A reproducible accelerated in vitro release testing method for PLGA microspheres
- PMID: 26705156
- PMCID: PMC4721604
- DOI: 10.1016/j.ijpharm.2015.12.031
A reproducible accelerated in vitro release testing method for PLGA microspheres
Abstract
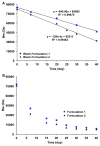
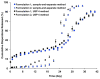
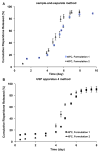
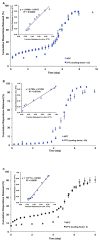
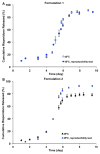
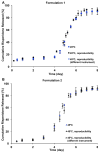
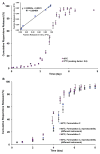
The objective of the present study was to develop a discriminatory and reproducible accelerated in vitro release method for long-acting PLGA microspheres with inner structure/porosity differences. Risperidone was chosen as a model drug. Qualitatively and quantitatively equivalent PLGA microspheres with different inner structure/porosity were obtained using different manufacturing processes. Physicochemical properties as well as degradation profiles of the prepared microspheres were investigated. Furthermore, in vitro release testing of the prepared risperidone microspheres was performed using the most common in vitro release methods (i.e., sample-and-separate and flow through) for this type of product. The obtained compositionally equivalent risperidone microspheres had similar drug loading but different inner structure/porosity. When microsphere particle size appeared similar, porous risperidone microspheres showed faster microsphere degradation and drug release compared with less porous microspheres. Both in vitro release methods investigated were able to differentiate risperidone microsphere formulations with differences in porosity under real-time (37 °C) and accelerated (45 °C) testing conditions. Notably, only the accelerated USP apparatus 4 method showed good reproducibility for highly porous risperidone microspheres. These results indicated that the accelerated USP apparatus 4 method is an appropriate fast quality control tool for long-acting PLGA microspheres (even with porous structures).
Keywords: Accelerated in vitro release; Compositionally equivalent; PLGA microspheres; Porous; Risperidone; USP apparatus 4.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Accelerated in vitro release testing method for naltrexone loaded PLGA microspheres.Int J Pharm. 2017 Mar 30;520(1-2):79-85. doi: 10.1016/j.ijpharm.2017.01.050. Epub 2017 Jan 30. Int J Pharm. 2017. PMID: 28153651
-
In vitro-in vivo correlation of parenteral risperidone polymeric microspheres.J Control Release. 2015 Nov 28;218:2-12. doi: 10.1016/j.jconrel.2015.09.051. Epub 2015 Sep 28. J Control Release. 2015. PMID: 26423236 Free PMC article.
-
Validation of USP apparatus 4 method for microsphere in vitro release testing using Risperdal Consta.Int J Pharm. 2011 Nov 28;420(2):198-205. doi: 10.1016/j.ijpharm.2011.08.035. Epub 2011 Aug 24. Int J Pharm. 2011. PMID: 21889583
-
Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications.J Control Release. 2014 Nov 10;193:324-40. doi: 10.1016/j.jconrel.2014.09.003. Epub 2014 Sep 10. J Control Release. 2014. PMID: 25218676 Review.
-
Recent advances in testing of microsphere drug delivery systems.Expert Opin Drug Deliv. 2016;13(4):593-608. doi: 10.1517/17425247.2016.1134484. Epub 2016 Feb 1. Expert Opin Drug Deliv. 2016. PMID: 26828874 Review.
Cited by
-
A review of current advancements for wound healing: Biomaterial applications and medical devices.J Biomed Mater Res B Appl Biomater. 2022 Nov;110(11):2542-2573. doi: 10.1002/jbm.b.35086. Epub 2022 May 17. J Biomed Mater Res B Appl Biomater. 2022. PMID: 35579269 Free PMC article. Review.
-
Recent Progress in Drug Release Testing Methods of Biopolymeric Particulate System.Pharmaceutics. 2021 Aug 23;13(8):1313. doi: 10.3390/pharmaceutics13081313. Pharmaceutics. 2021. PMID: 34452274 Free PMC article. Review.
-
Formulation composition, manufacturing process, and characterization of poly(lactide-co-glycolide) microparticles.J Control Release. 2021 Jan 10;329:1150-1161. doi: 10.1016/j.jconrel.2020.10.044. Epub 2020 Oct 24. J Control Release. 2021. PMID: 33148404 Free PMC article. Review.
-
In vitro dissolution considerations associated with nano drug delivery systems.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021 Nov;13(6):e1732. doi: 10.1002/wnan.1732. Epub 2021 Jun 15. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021. PMID: 34132050 Free PMC article. Review.
-
Challenges and Complications of Poly(lactic-co-glycolic acid)-Based Long-Acting Drug Product Development.Pharmaceutics. 2022 Mar 11;14(3):614. doi: 10.3390/pharmaceutics14030614. Pharmaceutics. 2022. PMID: 35335988 Free PMC article. Review.
References
-
- Alexis F. Factors affecting the degradation and drug-release mechanism of poly(lactic acid) and poly[(lactic acid)-co-(glycolic acid)] Polym Int. 2005;54:36–46.
-
- Chu DF, Fu XQ, Liu WH, Liu K, Li YX. Pharmacokinetics and in vitro and in vivo correlation of huperzine A loaded poly(lactic-co-glycolic acid) microspheres in dogs. Int J Pharm. 2006;325:116–123. - PubMed
-
- D’Souza SS, DeLuca PP. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm Res. 2006;23:460–474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

