Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax
- PMID: 26707935
- PMCID: PMC4874660
- DOI: 10.1038/leu.2015.350
Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax
Abstract
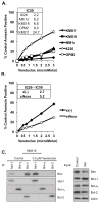
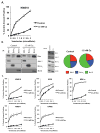
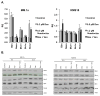
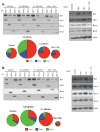
Venetoclax (ABT-199), a specific inhibitor of the anti-apoptotic protein Bcl-2, is currently in phase I clinical trials for multiple myeloma. The results suggest that venetoclax is only active in a small cohort of patients therefore we wanted to determine its efficacy when used in combination. Combining venetoclax with melphalan or carfilzomib produced additive or better cell death in four of the five cell lines tested. The most striking results were seen with dexamethasone (Dex). Co-treatment of human myeloma cell lines and primary patient samples, with Dex and venetoclax, significantly increased cell death over venetoclax alone in four of the five cell lines, and in all patient samples tested. The mechanism by which this occurs is an increase in the expression of both Bcl-2 and Bim upon addition of Dex. This results in alterations in Bim binding to anti-apoptotic proteins. Dex shifts Bim binding towards Bcl-2 resulting in increased sensitivity to venetoclax. These data suggest that knowledge of drug-induced alterations of Bim-binding patterns may help inform better combination drug regimens. Furthermore, the data indicate combining this novel therapeutic with Dex could be an effective therapy for a broader range of patients than would be predicted by single-agent activity.
Trial registration: ClinicalTrials.gov NCT01794507.
Figures


References
-
- Cancer Facts & Figures. Ameriican Cancer Society; 2015.
-
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006 May;9(5):351–365. - PubMed
-
- Kuroda J, Taniwaki M. Involvement of BH3-only proteins in hematologic malignancies. Crit Rev Oncol Hematol. 2009 Aug;71(2):89–101. - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

