Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis
- PMID: 26708108
- PMCID: PMC4908634
- DOI: 10.1038/npp.2015.367
Oral Cannabidiol does not Alter the Subjective, Reinforcing or Cardiovascular Effects of Smoked Cannabis
Abstract
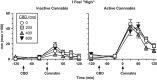
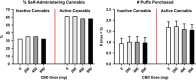
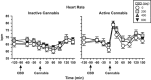
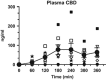
Cannabidiol (CBD), a constituent of cannabis with few psychoactive effects, has been reported in some studies to attenuate certain aspects of Δ(9)-tetrahydrocannabinol (THC) intoxication. However, most studies have tested only one dose of CBD in combination with one dose of oral THC, making it difficult to assess the nature of this interaction. Further, the effect of oral CBD on smoked cannabis administration is unknown. The objective of this multi-site, randomized, double-blind, within-subject laboratory study was to assess the influence of CBD (0, 200, 400, 800 mg, p.o.) pretreatment on the reinforcing, subjective, cognitive, and physiological effects of smoked cannabis (0.01 (inactive), 5.30-5.80% THC). Non-treatment-seeking, healthy cannabis smokers (n=31; 17M, 14 F) completed eight outpatient sessions. CBD was administered 90 min prior to cannabis administration. The behavioral and cardiovascular effects of cannabis were measured at baseline and repeatedly throughout the session. A subset of participants (n=8) completed an additional session to measure plasma CBD concentrations after administration of the highest CBD dose (800 mg). Under placebo CBD conditions, active cannabis (1) was self-administered by significantly more participants than placebo cannabis and (2) produced significant, time-dependent increases in ratings of 'High', 'Good Effect', ratings of the cannabis cigarette (eg, strength, liking), and heart rate relative to inactive cannabis. CBD, which alone produced no significant psychoactive or cardiovascular effects, did not significantly alter any of these outcomes. Cannabis self-administration, subjective effects, and cannabis ratings did not vary as a function of CBD dose relative to placebo capsules. These findings suggest that oral CBD does not reduce the reinforcing, physiological, or positive subjective effects of smoked cannabis.
Figures

References
-
- Agurell S, Carlsson S, Lindgren J, Ohlsson A, Gillespie H, Hollister L (1981). Interactions of Δ11-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentographywith cannabinol and cannabidiol following oral administration in man. Assay of cannab. Experientia 37: 1090–1092. - PubMed
-
- Agurell S, Haldin M, Lindgren J, Ohlsson A, Widman M, Gillespie H et al (1986). Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev 38: 21–43. - PubMed
-
- Benowitz NL, Nguyen T, Jones RT, Herning R, Bachman J (1980). Metabolic and psychophysiologic studies of cannabidiol-hexobarbital interaction. Clin Pharmacol Ther 28: 115–120. - PubMed
-
- Bergamaschi M, Queiroz R, Zuardi A, Crippa J (2011. b). safety and side effects of cannabidiol, a cannabis sativa constituent. Curr Drug Safety 6: 237–249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

