Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis
- PMID: 26708384
- PMCID: PMC4814243
- DOI: 10.1111/cas.12871
Wnt5a-Ror2 signaling in mesenchymal stem cells promotes proliferation of gastric cancer cells by activating CXCL16-CXCR6 axis
Abstract
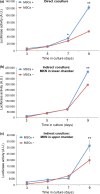
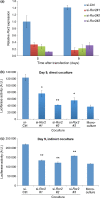
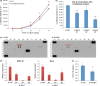
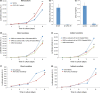
Wnt5a-Ror2 signaling has been shown to play important roles in promoting aggressiveness of various cancer cells in a cell-autonomous manner. However, little is known about its function in cancer-associated stromal cells, including mesenchymal stem cells (MSCs). Thus, we examined the role of Wnt5a-Ror2 signaling in bone marrow-derived MSCs in regulating proliferation of undifferentiated gastric cancer cells. Coculture of a gastric cancer cell line, MKN45, with MSCs either directly or indirectly promotes proliferation of MKN45 cells, and suppressed expression of Ror2 in MSCs prior to coculture inhibits enhanced proliferation of MKN45 cells. In addition, conditioned media from MSCs, treated with control siRNA, but not siRNAs against Ror2, can enhance proliferation of MKN45 cells. Interestingly, it was found that expression of CXCL16 in MSCs is augmented by Wnt5a-Ror2 signaling, and that recombinant chemokine (C-X-C motif) ligand (CXCL)16 protein can enhance proliferation of MKN45 cells in the absence of MSCs. In fact, suppressed expression of CXCL16 in MSCs or an addition of a neutralizing antibody against CXCL16 fails to promote proliferation of MKN45 cells in either direct or indirect coculture with MSCs. Importantly, we show that MKN45 cells express chemokine (C-X-C motif) receptor (CXCR)6, a receptor for CXCL16, and that suppressed expression of CXCR6 in MKN45 cells results in a failure of its enhanced proliferation in either direct or indirect coculture with MSCs. These findings indicate that Wnt5a-Ror2 signaling enhances expression of CXCL16 in MSCs and, as a result, enhanced secretion of CXCL16 from MSCs might act on CXCR6 expressed on MKN45, leading to the promotion of its proliferation.
Keywords: CXCL16; CXCR6; Wnt5a-Ror2 signaling; gastric cancer cells; mesenchymal stem cells (MSCs).
© 2015 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
Similar articles
-
Mesenchymal stem cell-derived CXCL16 promotes progression of gastric cancer cells by STAT3-mediated expression of Ror1.Cancer Sci. 2020 Apr;111(4):1254-1265. doi: 10.1111/cas.14339. Epub 2020 Feb 25. Cancer Sci. 2020. PMID: 32012403 Free PMC article.
-
Autonomous and intercellular chemokine signaling elicited from mesenchymal stem cells regulates migration of undifferentiated gastric cancer cells.Genes Cells. 2022 May;27(5):368-375. doi: 10.1111/gtc.12933. Epub 2022 Mar 16. Genes Cells. 2022. PMID: 35261108
-
Myeloma cells inhibit non-canonical wnt co-receptor ror2 expression in human bone marrow osteoprogenitor cells: effect of wnt5a/ror2 pathway activation on the osteogenic differentiation impairment induced by myeloma cells.Leukemia. 2013 Feb;27(2):451-63. doi: 10.1038/leu.2012.190. Epub 2012 Jul 11. Leukemia. 2013. PMID: 22781592
-
CXCR6/CXCL16 functions as a regulator in metastasis and progression of cancer.Biochim Biophys Acta. 2010 Aug;1806(1):42-9. doi: 10.1016/j.bbcan.2010.01.004. Epub 2010 Feb 1. Biochim Biophys Acta. 2010. PMID: 20122997 Review.
-
Cell/tissue-tropic functions of Wnt5a signaling in normal and cancer cells.Trends Cell Biol. 2010 Jun;20(6):346-54. doi: 10.1016/j.tcb.2010.03.001. Epub 2010 Mar 30. Trends Cell Biol. 2010. PMID: 20359892 Review.
Cited by
-
Inflammatory Human Umbilical Cord-Derived Mesenchymal Stem Cells Promote Stem Cell-Like Characteristics of Cancer Cells in an IL-1β-Dependent Manner.Biomed Res Int. 2018 Feb 18;2018:7096707. doi: 10.1155/2018/7096707. eCollection 2018. Biomed Res Int. 2018. PMID: 29670904 Free PMC article.
-
CXCL16 Promotes Gastric Cancer Tumorigenesis via ADAM10-Dependent CXCL16/CXCR6 Axis and Activates Akt and MAPK Signaling Pathways.Int J Biol Sci. 2021 Jul 5;17(11):2841-2852. doi: 10.7150/ijbs.57826. eCollection 2021. Int J Biol Sci. 2021. PMID: 34345211 Free PMC article.
-
The Chemokine Receptor CXCR6 Evokes Reverse Signaling via the Transmembrane Chemokine CXCL16.Int J Mol Sci. 2017 Jul 8;18(7):1468. doi: 10.3390/ijms18071468. Int J Mol Sci. 2017. PMID: 28698473 Free PMC article.
-
WNT Signaling in Stem Cells: A Look into the Non-Canonical Pathway.Stem Cell Rev Rep. 2024 Jan;20(1):52-66. doi: 10.1007/s12015-023-10610-5. Epub 2023 Oct 7. Stem Cell Rev Rep. 2024. PMID: 37804416 Free PMC article. Review.
-
Stemness in Cancer: Stem Cells, Cancer Stem Cells, and Their Microenvironment.Stem Cells Int. 2017;2017:5619472. doi: 10.1155/2017/5619472. Epub 2017 Apr 4. Stem Cells Int. 2017. PMID: 28473858 Free PMC article. Review.
References
-
- Oishi I, Suzuki H, Onishi N et al The receptor tyrosine kinase Ror2 is involved in non‐canonical Wnt5a/JNK signaling pathway. Genes Cells 2003; 8: 645–54. - PubMed
-
- Nishita M, Enomoto M, Yamagata K, Minami Y. Cell/tissue‐tropic functions of Wnt5a signaling in normal and cancer cells. Trends Cell Biol 2010; 20: 346–54. - PubMed
-
- Endo M, Nishita M, Fujii M, Minami Y. Insight into the role of Wnt5a‐induced signaling in normal and cancer cells. Int Rev Cell Mol Biol 2015; 314: 117–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

