Survey of Clinical Laboratory Practices for 2015 Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea
- PMID: 26709263
- PMCID: PMC4713849
- DOI: 10.3343/alm.2016.36.2.154
Survey of Clinical Laboratory Practices for 2015 Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea
Abstract
Background: It is crucial to understand the current status of clinical laboratory practices for the largest outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infections in the Republic of Korea to be well prepared for future emerging infectious diseases.
Methods: We conducted a survey of 49 clinical laboratories in medical institutions and referral medical laboratories. A short questionnaire to survey clinical laboratory practices relating to MERS-CoV diagnostic testing was sent by email to the directors and clinical pathologists in charge of the clinical laboratories performing MERS-CoV testing. The survey focused on testing volume, reporting of results, resources, and laboratory safety.
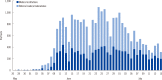
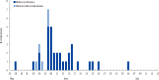
Results: A total of 40 clinical laboratories responded to the survey. A total of 27,009 MERS-CoV real-time reverse transcription PCR (rRT-PCR) tests were performed. Most of the specimens were sputum (73.5%). The median turnaround time (TAT) was 5.29 hr (first and third quartile, 4.11 and 7.48 hr) in 26 medical institutions. The median TAT of more than a half of the laboratories (57.7%) was less than 6 hr. Many laboratories were able to perform tests throughout the whole week. Laboratory biosafety preparedness included class II biosafety cabinets (100%); separated pre-PCR, PCR, and post-PCR rooms (88.6%); negative pressure pretreatment rooms (48.6%); and negative pressure sputum collection rooms (20.0%).
Conclusions: Clinical laboratories were able to quickly expand their diagnostic capacity in response to the 2015 MERS-CoV outbreak. Our results show that clinical laboratories play an important role in the maintenance and enhancement of laboratory response in preparation for future emerging infections.
Keywords: Clinical laboratory; Korea; Middle East respiratory syndrome coronavirus (MERS-CoV); Outbreak; Preparedness; Survey.
Conflict of interest statement
Figures
References
-
- Hayden RT, Wick MT, Rodriguez AB, Caliendo AM, Mitchell MJ, Ginocchio CC. A survey-based assessment of United States clinical laboratory response to the 2009 H1N1 influenza outbreak. Arch Pathol Lab Med. 2010;134:1671–1678. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources