Morin, a Bioflavonoid Suppresses Monosodium Urate Crystal-Induced Inflammatory Immune Response in RAW 264.7 Macrophages through the Inhibition of Inflammatory Mediators, Intracellular ROS Levels and NF-κB Activation
- PMID: 26709520
- PMCID: PMC4692533
- DOI: 10.1371/journal.pone.0145093
Morin, a Bioflavonoid Suppresses Monosodium Urate Crystal-Induced Inflammatory Immune Response in RAW 264.7 Macrophages through the Inhibition of Inflammatory Mediators, Intracellular ROS Levels and NF-κB Activation
Abstract
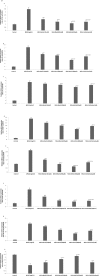
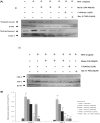
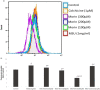
Our previous studies had reported that morin, a bioflavanoid exhibited potent anti-inflammatory effect against adjuvant-induced arthritic rats. In this current study, we investigated the anti-inflammatory mechanism of morin against monosodium urate crystal (MSU)-induced inflammation in RAW 264.7 macrophage cells, an in vitro model for acute gouty arthritis. For comparison purpose, colchicine was used as a reference drug. We have observed that morin (100-300 μM) treatment significantly suppressed the levels of inflammatory cytokines (TNF-α, IL-1β, IL-6, MCP-1 and VEGF), inflammatory mediators (NO and PEG2), and lysosomal enzymes (acid phosphatase, β-galactosidase, N-acetyl glucosamindase and cathepsin D) in MSU-crystals stimulated macrophage cells. The mRNA expression of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and MCP-1), inflammatory enzymes (iNOS and COX-2), and NF-κBp65 was found downregulated in MSU crystal stimulated macrophage cells by morin treatment, however, the mRNA expression of hypoxanthine phospho ribosyl transferse (HPRT) was found to be increased. The flow cytometry analysis revealed that morin treatment decreased intracellular reactive oxygen species levels in MSU crystal stimulated macrophage cells. The western blot analysis clearly showed that morin mainly exerts its anti-inflammatory effects by inhibiting the MSU crystal-induced COX-2 and TNF-α protein expression through the inactivation of NF-κB signaling pathway in RAW 264.7 macrophage cells similar to that of BAY 11-7082 (IκB kinase inhibitor). Our results collectively suggest that morin can be a potential therapeutic agent for inflammatory disorders like acute gouty arthritis.
Conflict of interest statement
Figures


References
-
- Liu-Bryan R, Scott P, Sydlaske A, Rose DM, Terkeltaub R (2005) Innate immunity conferred by Toll-like receptors 2 and 4 and myeloid differentiation factor 88 expression is pivotal to monosodium urate monohydrate crystal-induced inflammation. Arthritis Rheum. 52(9):2936–46. 10.1002/art.21238 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

