In Situ Analysis of a Silver Nanoparticle-Precipitating Shewanella Biofilm by Surface Enhanced Confocal Raman Microscopy
- PMID: 26709923
- PMCID: PMC4692441
- DOI: 10.1371/journal.pone.0145871
In Situ Analysis of a Silver Nanoparticle-Precipitating Shewanella Biofilm by Surface Enhanced Confocal Raman Microscopy
Erratum in
-
Correction: In Situ Analysis of a Silver Nanoparticle-Precipitating Shewanella Biofilm by Surface Enhanced Confocal Raman Microscopy.PLoS One. 2018 Jun 14;13(6):e0199344. doi: 10.1371/journal.pone.0199344. eCollection 2018. PLoS One. 2018. PMID: 29902257 Free PMC article.
Abstract
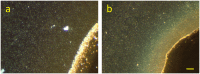
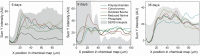
Shewanella oneidensis MR-1 is an electroactive bacterium, capable of reducing extracellular insoluble electron acceptors, making it important for both nutrient cycling in nature and microbial electrochemical technologies, such as microbial fuel cells and microbial electrosynthesis. When allowed to anaerobically colonize an Ag/AgCl solid interface, S. oneidensis has precipitated silver nanoparticles (AgNp), thus providing the means for a surface enhanced confocal Raman microscopy (SECRaM) investigation of its biofilm. The result is the in-situ chemical mapping of the biofilm as it developed over time, where the distribution of cytochromes, reduced and oxidized flavins, polysaccharides and phosphate in the undisturbed biofilm is monitored. Utilizing AgNp bio-produced by the bacteria colonizing the Ag/AgCl interface, we could perform SECRaM while avoiding the use of a patterned or roughened support or the introduction of noble metal salts and reducing agents. This new method will allow a spatially and temporally resolved chemical investigation not only of Shewanella biofilms at an insoluble electron acceptor, but also of other noble metal nanoparticle-precipitating bacteria in laboratory cultures or in complex microbial communities in their natural habitats.
Conflict of interest statement
Figures






References
-
- Lovley DR. Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol. 2006;17(3):327–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

