Natrelle Silicone Breast Implant Follow-Up Study: Demographics, Lifestyle, and Surgical Characteristics of More Than 50,000 Augmentation Subjects
- PMID: 26710009
- PMCID: PMC5412600
- DOI: 10.1097/PRS.0000000000001851
Natrelle Silicone Breast Implant Follow-Up Study: Demographics, Lifestyle, and Surgical Characteristics of More Than 50,000 Augmentation Subjects
Abstract
Background: A large, multicenter, 10-year observational study is being conducted to compare the long-term safety and effectiveness of Natrelle silicone breast implants with saline implants or national norms. Study baseline data and surgical characteristics are reported here.
Methods: Women seeking primary augmentation, revision-augmentation, primary reconstruction, or revision-reconstruction participated. Eligible subjects had completed surgery and received one implant or matching implants. Baseline demographics, health, lifestyle, and surgical characteristics were recorded. Data are presented here for subjects (≥22 years old) who underwent primary augmentation or revision-augmentation.
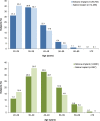
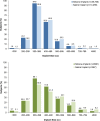
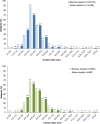
Results: Of 50,979 subjects who underwent augmentation procedures, 35,756 received silicone implants and 15,223 received saline implants. Of these, 86.3 percent underwent primary augmentation, and 13.7 percent underwent revision-augmentation; nearly all subjects (99.3 percent) received bilateral implants. In the primary augmentation group, 67.6 percent of subjects received silicone implants versus 86.1 percent in the revision-augmentation group. Median age was lower in the primary augmentation group compared with the revision-augmentation group (33 versus 42 years old, respectively). Most subjects were white nonsmokers and had attended college. Hispanic subjects and subjects with a body mass index of 25 kg/m or greater were more likely to receive saline versus silicone implants. Across groups, the most common characteristics by procedure or implant type included inframammary incision site (54.6 percent), partial (58.2 percent) or complete (31.9 percent) submuscular placement, smooth surface implants (93.1 percent), and implant size of 300 to 399 cc. Incision size was larger for silicone versus saline implants.
Conclusion: These data add to the body of knowledge on women undergoing augmentation procedures by providing an unprecedented look at a large number of subjects.
Trial registration: ClinicalTrials.gov NCT00443274.
Conflict of interest statement
Figures
Similar articles
-
Natrelle Silicone Breast Implant Follow-up Study: Demographics, Lifestyle, and Surgical Characteristics of More Than 5000 Reconstruction Subjects.Plast Reconstr Surg Glob Open. 2015 Aug 25;3(8):e489. doi: 10.1097/GOX.0000000000000406. eCollection 2015 Aug. Plast Reconstr Surg Glob Open. 2015. PMID: 26495202 Free PMC article.
-
Risk Factor Analysis for Capsular Contracture, Malposition, and Late Seroma in Subjects Receiving Natrelle 410 Form-Stable Silicone Breast Implants.Plast Reconstr Surg. 2017 Jan;139(1):1-9. doi: 10.1097/PRS.0000000000002837. Plast Reconstr Surg. 2017. PMID: 27627058 Free PMC article. Clinical Trial.
-
Five-Year Safety Data for More than 55,000 Subjects following Breast Implantation: Comparison of Rare Adverse Event Rates with Silicone Implants versus National Norms and Saline Implants.Plast Reconstr Surg. 2017 Oct;140(4):666-679. doi: 10.1097/PRS.0000000000003711. Plast Reconstr Surg. 2017. PMID: 28953716
-
Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type.J Plast Reconstr Aesthet Surg. 2013 Sep;66(9):1165-72. doi: 10.1016/j.bjps.2013.04.046. Epub 2013 May 9. J Plast Reconstr Aesthet Surg. 2013. PMID: 23664574 Review.
-
Clinical trial outcomes of high- and extra high-profile breast implants.Aesthet Surg J. 2013 May;33(4):529-39. doi: 10.1177/1090820X13484035. Epub 2013 Apr 4. Aesthet Surg J. 2013. PMID: 23559355 Review.
Cited by
-
Validation of the Vectra XT three-dimensional imaging system for measuring breast volume and symmetry following oncological reconstruction.Breast Cancer Res Treat. 2018 Sep;171(2):391-398. doi: 10.1007/s10549-018-4843-6. Epub 2018 Jun 5. Breast Cancer Res Treat. 2018. PMID: 29872939 Free PMC article.
-
A comparison of perioperative safety for breast augmentation in cis- vs. trans patients.Ann Transl Med. 2021 Apr;9(7):601. doi: 10.21037/atm-20-3355. Ann Transl Med. 2021. PMID: 33987299 Free PMC article.
-
Lactation Outcomes in More Than 3500 Women Following Primary Augmentation: 5-Year Data From the Breast Implant Follow-Up Study.Aesthet Surg J. 2019 Jul 12;39(8):875-883. doi: 10.1093/asj/sjy221. Aesthet Surg J. 2019. PMID: 30165661 Free PMC article.
References
-
- American Society of Plastic Surgeons. 2013 Plastic Surgery Statistics Report. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2013-s.... Accessed November 9, 2015.
-
- Jewell ML. Silicone gel breast implants at 50: The state of the science. Aesthet Surg J. 2012;32:1031–1034. - PubMed
-
- Centers for Devices and Radiological Health; U.S. Food and Drug Administration. FDA update on the safety of silicone gel-filled breast implants; June 2011. Available at: http://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures.... Accessed November 9, 2015.
-
- Allergan, Inc. Irvine, Calif.: Allergan, Inc.; 2009. Natrelle Silicone-Filled Breast Implants [directions for use].
-
- Reece EM, Ghavami A, Hoxworth RE, et al. Primary breast augmentation today: A survey of current breast augmentation practice patterns. Aesthet Surg J. 2009;29:116–121. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

