A Randomized Trial of Pharmacogenetic Warfarin Dosing in Naïve Patients with Non-Valvular Atrial Fibrillation
- PMID: 26710337
- PMCID: PMC4692529
- DOI: 10.1371/journal.pone.0145318
A Randomized Trial of Pharmacogenetic Warfarin Dosing in Naïve Patients with Non-Valvular Atrial Fibrillation
Abstract
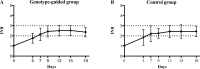
Genotype-guided warfarin dosing have been proposed to improve patient’s management. This study is aimed to determine whether a CYP2C9- VKORC1- CYP4F2-based pharmacogenetic algorithm is superior to a standard, clinically adopted, pharmacodynamic method. Two-hundred naïve patients with non-valvular atrial fibrillation were randomized to trial arms and 180 completed the study. No significant differences were found in the number of out-of-range INRs (INR<2.0 or >3.0) (p = 0.79) and in the mean percentage of time spent in the therapeutic range (TTR) after 19 days in the pharmacogenetic (51.9%) and in the control arm (53.2%, p = 0.71). The percentage of time spent at INR>4.0 was significantly lower in the pharmacogenetic (0.7%) than in the control arm (1.8%) (p = 0.02). Genotype-guided warfarin dosing is not superior in overall anticoagulation control when compared to accurate clinical standard of care.
Trial registration: ClinicalTrials.gov NCT01178034.
Conflict of interest statement
Figures


References
-
- Palareti G, Leali N, Coccheri S, Poggi M, Manotti C, D'Angelo A, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996; 348: 423–428. - PubMed
-
- Scordo M.G, Pengo V, Spina E, Dahl ML, Gusella M, Padrini R. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther. 2002; 72: 702–710. - PubMed
-
- D'Andrea G, D'Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, et al. A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood. 2005; 105: 645–649. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

