Treatment evolution for metastatic castration-resistant prostate cancer with recent introduction of novel agents: retrospective analysis of real-world data
- PMID: 26710718
- PMCID: PMC4735776
- DOI: 10.1002/cam4.576
Treatment evolution for metastatic castration-resistant prostate cancer with recent introduction of novel agents: retrospective analysis of real-world data
Abstract
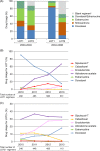
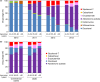
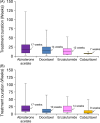
Despite increasing drug treatment options for metastatic castration-resistant prostate cancer (mCRPC) patients, real-world treatment data are lacking. We conducted retrospective analyses of commercial claims and electronic medical record (EMR) databases to understand how treatment patterns for mCRPC have changed in a US-based real-world population. Truven Health Analytics MarketScan(®) (2000-2013) and EMR (2004-2013) databases were used to identify patients with an index prostate cancer diagnosis (ICD-9 codes 185X or 233.4X) and prescription claims for an mCRPC drug (mitoxantrone, estramustine, docetaxel, sipuleucel-T, cabazitaxel, abiraterone acetate, enzalutamide, or radium-223). Regimen analyses for first line of therapy (LOT1), second line of therapy, and beyond were performed among cohorts based on year of first mCRPC drug usage. mCRPC drug usage and treatment duration were compared across cohorts and age groups within each cohort. The commercial claims cohort yielded 3437 evaluable patients. Most men (91%) commencing mCRPC treatment had docetaxel as LOT1 in 2010; this number had declined to 15% in 2013. In 2013, 67% and 9% of patients used abiraterone acetate and enzalutamide, respectively, as LOT1. Among both commercial claims and EMR cohorts, treatment pattern changes were most pronounced in men aged >80 years, and median treatment duration for some mCRPC drugs was shorter than expected based on available clinical trial information. These results demonstrate a shift in mCRPC treatments during the past 5 years, with greater use of newer noncytotoxic treatments than docetaxel. These real-world data aid in understanding the changing role of chemotherapy in the management of mCRPC.
Keywords: metastatic castration-resistant prostate cancer; prostate cancer; real-world; treatment patterns.
© 2015 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures

References
-
- Garnick, M. B. , Glode L. M., Smith J. A. Jr, and Max D. T.. 1985. Leuprolide: a review of its effects in comparison with diethylstilboestrol in the treatment of advanced cancer of the prostate. Br. J. Clin. Pract. 39:73–76. - PubMed
-
- Oudard, S. 2013. Progress in emerging therapies for advanced prostate cancer. Cancer Treat. Rev. 39:275–289. - PubMed
-
- Lam, J. S. , Leppert J. T., Vemulapalli S. N., Shvarts O., and Belldegrun A. S.. 2006. Secondary hormonal therapy for advanced prostate cancer. J. Urol. 175:27–34. - PubMed
-
- Tannock, I. F. , Osoba D., Stockler M. R., Ernst D. S., Neville A. J., Moore M. J., et al. 1996. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone‐resistant prostate cancer: a Canadian randomized trial with palliative end points. J. Clin. Oncol. 14:1756–1764. - PubMed
-
- Petrylak, D. P. , Tangen C. M., Hussain M. H., Lara P. N. Jr, Jones J. A., Taplin M. E., et al. 2004. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351:1513–1520. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

