p120RasGAP Protein Mediates Netrin-1 Protein-induced Cortical Axon Outgrowth and Guidance
- PMID: 26710849
- PMCID: PMC4813483
- DOI: 10.1074/jbc.M115.674846
p120RasGAP Protein Mediates Netrin-1 Protein-induced Cortical Axon Outgrowth and Guidance
Abstract
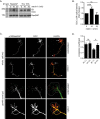
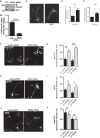
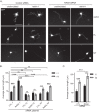
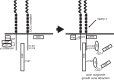
The receptor deleted in colorectal cancer (DCC) mediates the attraction of growing axons to netrin-1 during brain development. In response to netrin-1 stimulation, DCC becomes a signaling platform to recruit proteins that promote axon outgrowth and guidance. The Ras GTPase-activating protein (GAP) p120RasGAP inhibits Ras activity and mediates neurite retraction and growth cone collapse in response to repulsive guidance cues. Here we show an interaction between p120RasGAP and DCC that positively regulates netrin-1-mediated axon outgrowth and guidance in embryonic cortical neurons. In response to netrin-1, p120RasGAP is recruited to DCC in growth cones and forms a multiprotein complex with focal adhesion kinase and ERK. We found that Ras/ERK activities are elevated aberrantly in p120RasGAP-deficient neurons. Moreover, the expression of p120RasGAP Src homology 2 (SH2)-SH3-SH2 domains, which interact with the C-terminal tail of DCC, is sufficient to restore netrin-1-dependent axon outgrowth in p120RasGAP-deficient neurons. We provide a novel mechanism that exploits the scaffolding properties of the N terminus of p120RasGAP to tightly regulate netrin-1/DCC-dependent axon outgrowth and guidance.
Keywords: DCC; Ras protein; netrin-1; neurite outgrowth; neurobiology; neurodevelopment; signal transduction.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Lykissas M. G., Batistatou A. K., Charalabopoulos K. A., and Beris A. E. (2007) The role of neurotrophins in axonal growth, guidance, and regeneration. Curr. Neurovasc. Res. 4, 143–151 - PubMed
-
- Sánchez-Camacho C., and Bovolenta P. (2009) Emerging mechanisms in morphogen-mediated axon guidance. BioEssays 31, 1013–1025 - PubMed
-
- Lai Wing Sun K., Correia J. P., and Kennedy T. E. (2011) Netrins: versatile extracellular cues with diverse functions. Development 138, 2153–2169 - PubMed
-
- Keino-Masu K., Masu M., Hinck L., Leonardo E. D., Chan S. S., Culotti J. G., and Tessier-Lavigne M. (1996) Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

