Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex
- PMID: 26711118
- PMCID: PMC4707070
- DOI: 10.1016/j.neuron.2015.11.028
Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex
Abstract
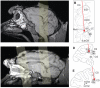
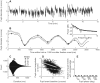
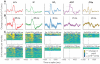
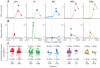
Changes in pupil diameter that reflect effort and other cognitive factors are often interpreted in terms of the activity of norepinephrine-containing neurons in the brainstem nucleus locus coeruleus (LC), but there is little direct evidence for such a relationship. Here, we show that LC activation reliably anticipates changes in pupil diameter that either fluctuate naturally or are driven by external events during near fixation, as in many psychophysical tasks. This relationship occurs on as fine a temporal and spatial scale as single spikes from single units. However, this relationship is not specific to the LC. Similar relationships, albeit with delayed timing and different reliabilities across sites, are evident in the inferior and superior colliculus and anterior and posterior cingulate cortex. Because these regions are interconnected with the LC, the results suggest that non-luminance-mediated changes in pupil diameter might reflect LC-mediated coordination of neuronal activity throughout some parts of the brain.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Comment in
-
More than Meets the Eye: the Relationship between Pupil Size and Locus Coeruleus Activity.Neuron. 2016 Jan 6;89(1):8-10. doi: 10.1016/j.neuron.2015.12.031. Neuron. 2016. PMID: 26748086 Free PMC article.
References
-
- Alnæs D, Sneve MH, Espeseth T, Endestad T, van de Pavert SH, Laeng B. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J Vis. 2014;14:1, 20. - PubMed
-
- Alpern M, Mason GL, Jardinco RE. Vergence and accommodation. V. Pupil size changes associated with changes in accommodative vergence. Am J Ophthalmol. 1961;52:762, 767. - PubMed
-
- Andreassi JL. Psychophysiology: Human behavior and physiological response. Lawrence Erlbaum Assoc.; Mahwah, NJ: 2000. Pupillary response and behavior; pp. 218–233.
-
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403, 450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

