Alternative Use of DNA Binding Domains by the Neurospora White Collar Complex Dictates Circadian Regulation and Light Responses
- PMID: 26711258
- PMCID: PMC4760224
- DOI: 10.1128/MCB.00841-15
Alternative Use of DNA Binding Domains by the Neurospora White Collar Complex Dictates Circadian Regulation and Light Responses
Abstract
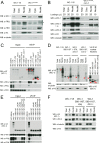
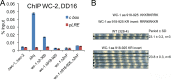
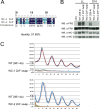
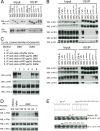
In the Neurospora circadian system, the White Collar complex (WCC) of WC-1 and WC-2 drives transcription of the circadian pacemaker gene frequency (frq), whose gene product, FRQ, as a part of the FRQ-FRH complex (FFC), inhibits its own expression. The WCC is also the principal Neurospora photoreceptor; WCC-mediated light induction of frq resets the clock, and all acute light induction is triggered by WCC binding to promoters of light-induced genes. However, not all acutely light-induced genes are also clock regulated, and conversely, not all clock-regulated direct targets of WCC are light induced; the structural determinants governing the shift from WCC's dark circadian role to its light activation role are poorly described. We report that the DBD region (named for being defective in binding DNA), a basic region in WC-1 proximal to the DNA-binding zinc finger (ZnF) whose function was previously ascribed to nuclear localization, instead plays multiple essential roles assisting in DNA binding and mediating interactions with the FFC. DNA binding for light induction by the WCC requires only WC-2, whereas DNA binding for circadian functions requires WC-2 as well as the ZnF and DBD motif of WC-1. The data suggest a means by which alterations in the tertiary and quaternary structures of the WCC can lead to its distinct functions in the dark and in the light.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
FRQ-interacting RNA helicase mediates negative and positive feedback in the Neurospora circadian clock.Genetics. 2010 Feb;184(2):351-61. doi: 10.1534/genetics.109.111393. Epub 2009 Nov 30. Genetics. 2010. PMID: 19948888 Free PMC article.
-
White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter.Science. 2002 Aug 2;297(5582):815-9. doi: 10.1126/science.1073681. Epub 2002 Jul 4. Science. 2002. PMID: 12098706
-
Domains required for the interaction of the central negative element FRQ with its transcriptional activator WCC within the core circadian clock of Neurospora.J Biol Chem. 2023 Jul;299(7):104850. doi: 10.1016/j.jbc.2023.104850. Epub 2023 May 21. J Biol Chem. 2023. PMID: 37220856 Free PMC article.
-
The molecular workings of the Neurospora biological clock.Novartis Found Symp. 2003;253:184-98; discussion 102-9, 198-202, 281-4. Novartis Found Symp. 2003. PMID: 14712922 Review.
-
Posttranslational regulation of Neurospora circadian clock by CK1a-dependent phosphorylation.Cold Spring Harb Symp Quant Biol. 2007;72:177-83. doi: 10.1101/sqb.2007.72.025. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419275 Review.
Cited by
-
Broad Substrate-Specific Phosphorylation Events Are Associated With the Initial Stage of Plant Cell Wall Recognition in Neurospora crassa.Front Microbiol. 2019 Nov 1;10:2317. doi: 10.3389/fmicb.2019.02317. eCollection 2019. Front Microbiol. 2019. PMID: 31736884 Free PMC article.
-
Transcriptome sequencing and global analysis of blue light-responsive genes provide clues for high carotenoid yields in Blakeslea trispora.Int Microbiol. 2022 May;25(2):325-338. doi: 10.1007/s10123-021-00225-6. Epub 2021 Nov 8. Int Microbiol. 2022. PMID: 34746983
-
WC-1 and the Proximal GATA Sequence Mediate a Cis-/Trans-Acting Repressive Regulation of Light-Dependent Gene Transcription in the Dark.Int J Mol Sci. 2019 Jun 12;20(12):2854. doi: 10.3390/ijms20122854. Int J Mol Sci. 2019. PMID: 31212732 Free PMC article.
-
The Phospho-Code Determining Circadian Feedback Loop Closure and Output in Neurospora.Mol Cell. 2019 May 16;74(4):771-784.e3. doi: 10.1016/j.molcel.2019.03.003. Epub 2019 Apr 3. Mol Cell. 2019. PMID: 30954403 Free PMC article.
-
Functional analysis of 110 phosphorylation sites on the circadian clock protein FRQ identifies clusters determining period length and temperature compensation.G3 (Bethesda). 2023 Feb 9;13(2):jkac334. doi: 10.1093/g3journal/jkac334. G3 (Bethesda). 2023. PMID: 36537198 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
