Requirement of Phosphoinositides Containing Stearic Acid To Control Cell Polarity
- PMID: 26711260
- PMCID: PMC4760214
- DOI: 10.1128/MCB.00843-15
Requirement of Phosphoinositides Containing Stearic Acid To Control Cell Polarity
Abstract
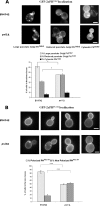
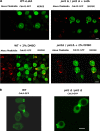
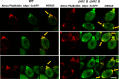
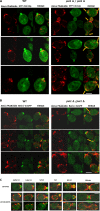
Phosphoinositides (PIPs) are present in very small amounts but are essential for cell signaling, morphogenesis, and polarity. By mass spectrometry, we demonstrated that some PIPs with stearic acyl chains were strongly disturbed in a psi1Δ Saccharomyces cerevisiae yeast strain deficient in the specific incorporation of a stearoyl chain at the sn-1 position of phosphatidylinositol. The absence of PIPs containing stearic acid induced disturbances in intracellular trafficking, although the total amount of PIPs was not diminished. Changes in PIPs also induced alterations in the budding pattern and defects in actin cytoskeleton organization (cables and patches). Moreover, when the PSI1 gene was impaired, a high proportion of cells with bipolar cortical actin patches that occurred concomitantly with the bipolar localization of Cdc42p was specifically found among diploid cells. This bipolar cortical actin phenotype, never previously described, was also detected in a bud9Δ/bud9Δ strain. Very interestingly, overexpression of PSI1 reversed this phenotype.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures









Similar articles
-
PSI1 is responsible for the stearic acid enrichment that is characteristic of phosphatidylinositol in yeast.FEBS J. 2009 Nov;276(21):6412-24. doi: 10.1111/j.1742-4658.2009.07355.x. Epub 2009 Oct 1. FEBS J. 2009. PMID: 19796168
-
Phosphoinositides containing stearic acid are required for interaction between Rho GTPases and the exocyst to control the late steps of polarized exocytosis.Traffic. 2022 Feb;23(2):120-136. doi: 10.1111/tra.12829. Epub 2022 Jan 10. Traffic. 2022. PMID: 34908215
-
The Saccharomyces cerevisiae Arf3 protein is involved in actin cable and cortical patch formation.FEMS Yeast Res. 2007 Sep;7(6):782-95. doi: 10.1111/j.1567-1364.2007.00239.x. Epub 2007 Apr 10. FEMS Yeast Res. 2007. PMID: 17425670
-
Phosphoinositides and Cell Polarity in the Drosophila Egg Chamber.Results Probl Cell Differ. 2017;63:169-187. doi: 10.1007/978-3-319-60855-6_8. Results Probl Cell Differ. 2017. PMID: 28779318 Review.
-
Phosphoinositides in yeast: genetically tractable signalling.FEMS Yeast Res. 2001 Apr;1(1):9-13. doi: 10.1111/j.1567-1364.2001.tb00008.x. FEMS Yeast Res. 2001. PMID: 12702458 Review.
Cited by
-
Phospholipid turnover and acyl chain remodeling in the yeast ER.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Jan;1865(1):158462. doi: 10.1016/j.bbalip.2019.05.006. Epub 2019 May 27. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31146038 Free PMC article. Review.
-
Fatty-acyl chain profiles of cellular phosphoinositides.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 May;1862(5):513-522. doi: 10.1016/j.bbalip.2017.02.002. Epub 2017 Feb 9. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28189644 Free PMC article.
-
Membrane curvature allosterically regulates the phosphatidylinositol cycle, controlling its rate and acyl-chain composition of its lipid intermediates.J Biol Chem. 2018 Nov 16;293(46):17780-17791. doi: 10.1074/jbc.RA118.005293. Epub 2018 Sep 20. J Biol Chem. 2018. PMID: 30237168 Free PMC article.
-
Emergence of Dip2-mediated specific DAG-based PKC signalling axis in eukaryotes.Elife. 2025 May 6;14:RP104011. doi: 10.7554/eLife.104011. Elife. 2025. PMID: 40327034 Free PMC article.
-
Phosphatidylinositol 4,5-bisphosphate (PIP2) and Ca2+ are both required to open the Cl- channel TMEM16A.J Biol Chem. 2019 Aug 16;294(33):12556-12564. doi: 10.1074/jbc.RA118.007128. Epub 2019 Jul 2. J Biol Chem. 2019. PMID: 31266809 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
