A Switch in Akt Isoforms Is Required for Notch-Induced Snail1 Expression and Protection from Cell Death
- PMID: 26711268
- PMCID: PMC4810475
- DOI: 10.1128/MCB.01074-15
A Switch in Akt Isoforms Is Required for Notch-Induced Snail1 Expression and Protection from Cell Death
Abstract
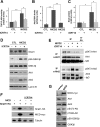
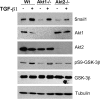
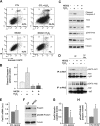
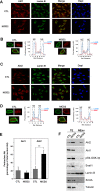
Notch activation in aortic endothelial cells (ECs) takes place at embryonic stages during cardiac valve formation and induces endothelial-to-mesenchymal transition (EndMT). Using aortic ECs, we show here that active Notch expression promotes EndMT, resulting in downregulation of vascular endothelial cadherin (VE-cadherin) and upregulation of mesenchymal genes such as those for fibronectin and Snail1/2. In these cells, transforming growth factor β1 exacerbates Notch effects by increasing Snail1 and fibronectin activation. When Notch-downstream pathways were analyzed, we detected an increase in glycogen synthase kinase 3β (GSK-3β) phosphorylation and inactivation that facilitates Snail1 nuclear retention and protein stabilization. However, the total activity of Akt was downregulated. The discrepancy between Akt activity and GSK-3β phosphorylation is explained by a Notch-induced switch in the Akt isoforms, whereby Akt1, the predominant isoform expressed in ECs, is decreased and Akt2 transcription is upregulated. Mechanistically, Akt2 induction requires the stimulation of the β-catenin/TCF4 transcriptional complex, which activates the Akt2 promoter. Active, phosphorylated Akt2 translocates to the nucleus in Notch-expressing cells, resulting in GSK-3β inactivation in this compartment. Akt2, but not Akt1, colocalizes in the nucleus with lamin B in the nuclear envelope. In addition to promoting GSK-3β inactivation, Notch downregulates Forkhead box O1 (FoxO1), another Akt2 nuclear substrate. Moreover, Notch protects ECs from oxidative stress-induced apoptosis through an Akt2- and Snail1-dependent mechanism.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Troglitazone ameliorates high glucose-induced EMT and dysfunction of SGLTs through PI3K/Akt, GSK-3β, Snail1, and β-catenin in renal proximal tubule cells.Am J Physiol Renal Physiol. 2010 May;298(5):F1263-75. doi: 10.1152/ajprenal.00475.2009. Epub 2009 Dec 16. Am J Physiol Renal Physiol. 2010. PMID: 20015942
-
Akt2 mediates TGF-β1-induced epithelial to mesenchymal transition by deactivating GSK3β/snail signaling pathway in renal tubular epithelial cells.Cell Physiol Biochem. 2014;34(2):368-82. doi: 10.1159/000363006. Epub 2014 Jul 11. Cell Physiol Biochem. 2014. PMID: 25059120
-
Stabilization of Snail through AKT/GSK-3β signaling pathway is required for TNF-α-induced epithelial-mesenchymal transition in prostate cancer PC3 cells.Eur J Pharmacol. 2013 Aug 15;714(1-3):48-55. doi: 10.1016/j.ejphar.2013.05.046. Epub 2013 Jun 11. Eur J Pharmacol. 2013. PMID: 23769744
-
The cross-talk of NOTCH and GSK-3 signaling in colon and other cancers.Biochim Biophys Acta Mol Cell Res. 2020 Sep;1867(9):118738. doi: 10.1016/j.bbamcr.2020.118738. Epub 2020 May 8. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 32389646 Review.
-
Akt1 in endothelial cell and angiogenesis.Cell Cycle. 2006 Mar;5(5):512-8. doi: 10.4161/cc.5.5.2538. Epub 2006 Mar 1. Cell Cycle. 2006. PMID: 16552185 Free PMC article. Review.
Cited by
-
Overview of Evidence-Based Chemotherapy for Oral Cancer: Focus on Drug Resistance Related to the Epithelial-Mesenchymal Transition.Biomolecules. 2021 Jun 16;11(6):893. doi: 10.3390/biom11060893. Biomolecules. 2021. PMID: 34208465 Free PMC article. Review.
-
A Computational Model of the Endothelial to Mesenchymal Transition.Front Genet. 2020 Mar 12;11:40. doi: 10.3389/fgene.2020.00040. eCollection 2020. Front Genet. 2020. PMID: 32226439 Free PMC article.
-
Isoflurane Promotes Non-Small Cell Lung Cancer Malignancy by Activating the Akt-Mammalian Target of Rapamycin (mTOR) Signaling Pathway.Med Sci Monit. 2016 Nov 29;22:4644-4650. doi: 10.12659/msm.898434. Med Sci Monit. 2016. PMID: 27897153 Free PMC article.
-
RUNX3 modulates hypoxia-induced endothelial-to-mesenchymal transition of human cardiac microvascular endothelial cells.Int J Mol Med. 2017 Jul;40(1):65-74. doi: 10.3892/ijmm.2017.2998. Epub 2017 May 22. Int J Mol Med. 2017. PMID: 28534977 Free PMC article.
-
Snail1: A Transcriptional Factor Controlled at Multiple Levels.J Clin Med. 2019 May 28;8(6):757. doi: 10.3390/jcm8060757. J Clin Med. 2019. PMID: 31141910 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
