Bidirectional Control of Synaptic GABAAR Clustering by Glutamate and Calcium
- PMID: 26711343
- PMCID: PMC4700050
- DOI: 10.1016/j.celrep.2015.12.002
Bidirectional Control of Synaptic GABAAR Clustering by Glutamate and Calcium
Abstract
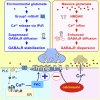
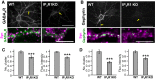
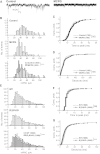
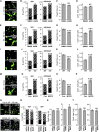
GABAergic synaptic transmission regulates brain function by establishing the appropriate excitation-inhibition (E/I) balance in neural circuits. The structure and function of GABAergic synapses are sensitive to destabilization by impinging neurotransmitters. However, signaling mechanisms that promote the restorative homeostatic stabilization of GABAergic synapses remain unknown. Here, by quantum dot single-particle tracking, we characterize a signaling pathway that promotes the stability of GABAA receptor (GABAAR) postsynaptic organization. Slow metabotropic glutamate receptor signaling activates IP3 receptor-dependent calcium release and protein kinase C to promote GABAAR clustering and GABAergic transmission. This GABAAR stabilization pathway counteracts the rapid cluster dispersion caused by glutamate-driven NMDA receptor-dependent calcium influx and calcineurin dephosphorylation, including in conditions of pathological glutamate toxicity. These findings show that glutamate activates distinct receptors and spatiotemporal patterns of calcium signaling for opposing control of GABAergic synapses.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Bannai H., Lévi S., Schweizer C., Dahan M., Triller A. Imaging the lateral diffusion of membrane molecules with quantum dots. Nat. Protoc. 2006;1:2628–2634. - PubMed
-
- Bannai H., Lévi S., Schweizer C., Inoue T., Launey T., Racine V., Sibarita J.B., Mikoshiba K., Triller A. Activity-dependent tuning of inhibitory neurotransmission based on GABAAR diffusion dynamics. Neuron. 2009;62:670–682. - PubMed
-
- Berridge M.J. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Bouthour W., Leroy F., Emmanuelli C., Carnaud M., Dahan M., Poncer J.C., Lévi S. A human mutation in Gabrg2 associated with generalized epilepsy alters the membrane dynamics of GABAA receptors. Cereb. Cortex. 2012;22:1542–1553. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

