Disrupted PI3K p110δ Signaling Dysregulates Maternal Immune Cells and Increases Fetal Mortality In Mice
- PMID: 26711346
- PMCID: PMC4700049
- DOI: 10.1016/j.celrep.2015.11.050
Disrupted PI3K p110δ Signaling Dysregulates Maternal Immune Cells and Increases Fetal Mortality In Mice
Abstract
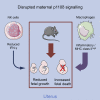
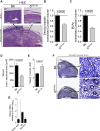
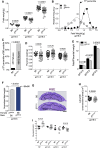
Maternal immune cells are an integral part of reproduction, but how they might cause pregnancy complications remains elusive. Macrophages and their dual function in inflammation and tissue repair are thought to play key yet undefined roles. Altered perinatal growth underpins adult morbidity, and natural killer (NK) cells may sustain fetal growth by establishing the placental blood supply. Using a mouse model of genetic inactivation of PI3K p110δ, a key intracellular signaling molecule in leukocytes, we show that p110δ regulates macrophage dynamics and NK-cell-mediated arterial remodeling. The uterus of dams with inactive p110δ had decreased IFN-γ and MHC class II(low) macrophages but enhanced IL-6. Poor vascular remodeling and a pro-inflammatory uterine milieu resulted in fetal death or growth retardation. Our results provide one mechanism that explains how imbalanced adaptations of maternal innate immune cells to gestation affect offspring well-being with consequence perinatally and possibly into adulthood.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Phosphoinositide 3-Kinase (PI3K) Subunit p110δ Is Essential for Trophoblast Cell Differentiation and Placental Development in Mouse.Sci Rep. 2016 Jun 16;6:28201. doi: 10.1038/srep28201. Sci Rep. 2016. PMID: 27306493 Free PMC article.
-
p110gamma and p110delta isoforms of phosphoinositide 3-kinase differentially regulate natural killer cell migration in health and disease.Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5795-800. doi: 10.1073/pnas.0808594106. Epub 2009 Mar 18. Proc Natl Acad Sci U S A. 2009. PMID: 19297623 Free PMC article.
-
p110delta is required for innate immunity to transplantable lymphomas.Biochem Soc Trans. 2007 Apr;35(Pt 2):183-5. doi: 10.1042/BST0350183. Biochem Soc Trans. 2007. PMID: 17371233
-
Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy.Semin Immunol. 2001 Aug;13(4):235-41. doi: 10.1006/smim.2000.0319. Semin Immunol. 2001. PMID: 11437631 Review.
-
Physiological role of IL-15 and IL-18 at the maternal-fetal interface.Chem Immunol Allergy. 2005;89:10-25. doi: 10.1159/000087906. Chem Immunol Allergy. 2005. PMID: 16129949 Review.
Cited by
-
Inhibition of Heme Oxygenase-1 by Zinc Protoporphyrin IX Improves Adverse Pregnancy Outcomes in Malaria During Early Gestation.Front Immunol. 2022 May 10;13:879158. doi: 10.3389/fimmu.2022.879158. eCollection 2022. Front Immunol. 2022. PMID: 35619717 Free PMC article.
-
Class IA PI3Ks regulate subcellular and functional dynamics of IDO1.EMBO Rep. 2020 Dec 3;21(12):e49756. doi: 10.15252/embr.201949756. Epub 2020 Nov 7. EMBO Rep. 2020. PMID: 33159421 Free PMC article.
-
Genetic Association between Swine Leukocyte Antigen Class II Haplotypes and Reproduction Traits in Microminipigs.Cells. 2019 Jul 26;8(8):783. doi: 10.3390/cells8080783. Cells. 2019. PMID: 31357541 Free PMC article.
-
Phosphoinositide 3-Kinase (PI3K) Subunit p110δ Is Essential for Trophoblast Cell Differentiation and Placental Development in Mouse.Sci Rep. 2016 Jun 16;6:28201. doi: 10.1038/srep28201. Sci Rep. 2016. PMID: 27306493 Free PMC article.
-
Beyond Maternal Tolerance: Education of Uterine Natural Killer Cells by Maternal MHC Drives Fetal Growth.Front Immunol. 2022 May 10;13:808227. doi: 10.3389/fimmu.2022.808227. eCollection 2022. Front Immunol. 2022. PMID: 35619712 Free PMC article.
References
-
- Aksoy E., Taboubi S., Torres D., Delbauve S., Hachani A., Whitehead M.A., Pearce W.P., Berenjeno I.M., Nock G., Filloux A. The p110δ isoform of the kinase PI(3)K controls the subcellular compartmentalization of TLR4 signaling and protects from endotoxic shock. Nat. Immunol. 2012;13:1045–1054. - PMC - PubMed
-
- Ashkar A.A., Croy B.A. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol. Reprod. 1999;61:493–502. - PubMed
-
- Ashkar A.A., Croy B.A. Functions of uterine natural killer cells are mediated by interferon gamma production during murine pregnancy. Semin. Immunol. 2001;13:235–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

