Pharmacokinetics of Transfer of Azithromycin into the Breast Milk of African Mothers
- PMID: 26711756
- PMCID: PMC4775927
- DOI: 10.1128/AAC.02668-15
Pharmacokinetics of Transfer of Azithromycin into the Breast Milk of African Mothers
Abstract
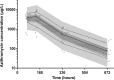
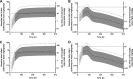
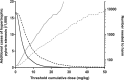
Azithromycin (AZI) is used for its antibiotic and antimalarial properties in pregnancy. Reported estimates of AZI breast milk transfer, based on concentrations in mostly single samples from small numbers of women, have suggested that infant intake is safe. To better characterize infant intake and the associated potential benefits and risks, AZI was measured by liquid chromatography-mass spectrometry in four breast milk samples taken over 28 days postpartum from each of 20 Gambian women given 2 g AZI during labor. A population pharmacokinetic model utilizing published parameters for AZI disposition in pregnancy, the present breast milk concentrations, and increasing/decreasing sigmoid maximum-effect (Emax) functions adequately described temporal changes in the milk/plasma ratio. The median estimated absolute and relative cumulative infant doses were 4.5 mg/kg of body weight (95% prediction interval, 0.6 to 7.0 mg/kg) and 15.7% (95% prediction interval, 2.0 to 27.8%) of the maternal dose, respectively; the latter exceeded the recommended 10% safety limit. Although some infants with bacterial infections may benefit from AZI in breast milk, there is a risk of hypertrophic pyloric stenosis with a worst-case number needed to harm of 60 based on the present and available epidemiologic data. (This study has been registered at ClinicalTrials.gov under registration no. NCT01800942.).
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


References
-
- Tiwari T, Murphy TV, Moran J. National Immunization Program CDC. 2005. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC Guidelines. MMWR Recomm Rep 54(RR-14):1–16. - PubMed
-
- Salman S, Rogerson SJ, Kose K, Griffin S, Gomorai S, Baiwog F, Winmai J, Kandai J, Karunajeewa HA, O'Halloran SJ, Siba P, Ilett KF, Mueller I, Davis TM. 2010. Pharmacokinetic properties of azithromycin in pregnancy. Antimicrob Agents Chemother 54:360–366. doi:10.1128/AAC.00771-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

