Suppression of Emergence of Resistance in Pathogenic Bacteria: Keeping Our Powder Dry, Part 2
- PMID: 26711766
- PMCID: PMC4775956
- DOI: 10.1128/AAC.02231-15
Suppression of Emergence of Resistance in Pathogenic Bacteria: Keeping Our Powder Dry, Part 2
Abstract
We are in a crisis of bacterial resistance. For economic reasons, most pharmaceutical companies are abandoning antimicrobial discovery efforts, while, in health care itself, infection control and antibiotic stewardship programs have generally failed to prevent the spread of drug-resistant bacteria. At this point, what can be done? The first step has been taken. Governments and international bodies have declared there is a worldwide crisis in antibiotic drug resistance. As discovery efforts begin anew, what more can be done to protect newly developing agents and improve the use of new drugs to suppress resistance emergence? A neglected path has been the use of recent knowledge regarding antibiotic dosing as single agents and in combination to minimize resistance emergence, while also providing sufficient early bacterial kill. In this review, we look at the data for resistance suppression. Approaches include increasing the intensity of therapy to suppress resistant subpopulations; developing concepts of clinical breakpoints to include issues surrounding suppression of resistance; and paying attention to the duration of therapy, which is another important issue for resistance suppression. New understanding of optimizing combination therapy is of interest for difficult-to-treat pathogens like Pseudomonas aeruginosa, Acinetobacter spp., and multidrug-resistant (MDR) Enterobacteriaceae. These lessons need to be applied to our old drugs as well to preserve them and to be put into national and international antibiotic resistance strategies. As importantly, from a regulatory perspective, new chemical entities should have a resistance suppression plan at the time of regulatory review. In this way, we can make the best of our current situation and improve future prospects.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

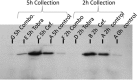
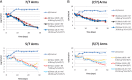
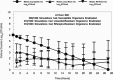
References
-
- Jumbe N, Louie A, Leary R, Liu W, Deziel MR, Tam VH, Bachhawat R, Freeman C, Kahn JB, Bush K, Dudley MN, Miller MH, Drusano GL. 2003. Application of a mathematical model to prevent in vivo amplification of antibiotic-resistant bacterial populations during therapy. J Clin Invest 112:275–285. doi:10.1172/JCI200316814. - DOI - PMC - PubMed
-
- Louie A, Brown DL, Liu W, Kulawy RW, Deziel MR, Drusano GL. 2007. In vitro infection model characterizing the effect of efflux pump inhibition on prevention of resistance to levofloxacin and ciprofloxacin in Streptococcus pneumoniae. Antimicrob Agents Chemother 51:3988–4000. doi:10.1128/AAC.00391-07. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

