Pan-neuronal imaging in roaming Caenorhabditis elegans
- PMID: 26711989
- PMCID: PMC4776525
- DOI: 10.1073/pnas.1507109113
Pan-neuronal imaging in roaming Caenorhabditis elegans
Abstract
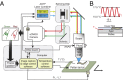
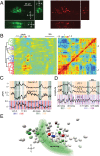
We present an imaging system for pan-neuronal recording in crawling Caenorhabditis elegans. A spinning disk confocal microscope, modified for automated tracking of the C. elegans head ganglia, simultaneously records the activity and position of ∼80 neurons that coexpress cytoplasmic calcium indicator GCaMP6s and nuclear localized red fluorescent protein at 10 volumes per second. We developed a behavioral analysis algorithm that maps the movements of the head ganglia to the animal's posture and locomotion. Image registration and analysis software automatically assigns an index to each nucleus and calculates the corresponding calcium signal. Neurons with highly stereotyped positions can be associated with unique indexes and subsequently identified using an atlas of the worm nervous system. To test our system, we analyzed the brainwide activity patterns of moving worms subjected to thermosensory inputs. We demonstrate that our setup is able to uncover representations of sensory input and motor output of individual neurons from brainwide dynamics. Our imaging setup and analysis pipeline should facilitate mapping circuits for sensory to motor transformation in transparent behaving animals such as C. elegans and Drosophila larva.
Keywords: C. elegans; Drosophila; calcium imaging; thermotaxis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Grabbing brain activity on the go.Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):1965-7. doi: 10.1073/pnas.1524219113. Epub 2016 Feb 3. Proc Natl Acad Sci U S A. 2016. PMID: 26842834 Free PMC article. No abstract available.
References
-
- Kawano T, et al. An imbalancing act: Gap junctions reduce the backward motor circuit activity to bias C. elegans for forward locomotion. Neuron. 2011;72(4):572–586. - PubMed
-
- Suzuki H, et al. In vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 2003;39(6):1005–1017. - PubMed
-
- Chalasani SH, et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450(7166):63–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

