One-carbon chemistry of oxalate oxidoreductase captured by X-ray crystallography
- PMID: 26712008
- PMCID: PMC4720323
- DOI: 10.1073/pnas.1518537113
One-carbon chemistry of oxalate oxidoreductase captured by X-ray crystallography
Abstract
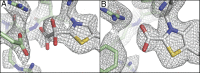
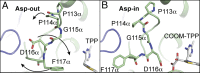
Thiamine pyrophosphate (TPP)-dependent oxalate oxidoreductase (OOR) metabolizes oxalate, generating two molecules of CO2 and two low-potential electrons, thus providing both the carbon and reducing equivalents for operation of the Wood-Ljungdahl pathway of acetogenesis. Here we present structures of OOR in which two different reaction intermediate bound states have been trapped: the covalent adducts between TPP and oxalate and between TPP and CO2. These structures, along with the previously determined structure of substrate-free OOR, allow us to visualize how active site rearrangements can drive catalysis. Our results suggest that OOR operates via a bait-and-switch mechanism, attracting substrate into the active site through the presence of positively charged and polar residues, and then altering the electrostatic environment through loop and side chain movements to drive catalysis. This simple but elegant mechanism explains how oxalate, a molecule that humans and most animals cannot break down, can be used for growth by acetogenic bacteria.
Keywords: carbon dioxide; oxalate; oxidoreductase; thiamine pyrophosphate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A structural phylogeny for understanding 2-oxoacid oxidoreductase function.Curr Opin Struct Biol. 2016 Dec;41:54-61. doi: 10.1016/j.sbi.2016.05.011. Epub 2016 Jun 14. Curr Opin Struct Biol. 2016. PMID: 27315560 Free PMC article. Review.
-
Identification and characterization of oxalate oxidoreductase, a novel thiamine pyrophosphate-dependent 2-oxoacid oxidoreductase that enables anaerobic growth on oxalate.J Biol Chem. 2010 Dec 24;285(52):40515-24. doi: 10.1074/jbc.M110.155739. Epub 2010 Oct 18. J Biol Chem. 2010. PMID: 20956531 Free PMC article.
-
The Structure of an Oxalate Oxidoreductase Provides Insight into Microbial 2-Oxoacid Metabolism.Biochemistry. 2015 Jul 7;54(26):4112-20. doi: 10.1021/acs.biochem.5b00521. Epub 2015 Jun 24. Biochemistry. 2015. PMID: 26061898 Free PMC article.
-
Properties of Intermediates in the Catalytic Cycle of Oxalate Oxidoreductase and Its Suicide Inactivation by Pyruvate.Biochemistry. 2017 Jun 6;56(22):2824-2835. doi: 10.1021/acs.biochem.7b00222. Epub 2017 May 23. Biochemistry. 2017. PMID: 28514140 Free PMC article.
-
The enzymes of oxalate metabolism: unexpected structures and mechanisms.Arch Biochem Biophys. 2005 Jan 1;433(1):176-92. doi: 10.1016/j.abb.2004.08.032. Arch Biochem Biophys. 2005. PMID: 15581576 Review.
Cited by
-
A reverse TCA cycle 2-oxoacid:ferredoxin oxidoreductase that makes C-C bonds from CO2.Joule. 2019 Feb 20;3(2):595-611. doi: 10.1016/j.joule.2018.12.006. Epub 2019 Jan 4. Joule. 2019. PMID: 31080943 Free PMC article.
-
Structural organization of pyruvate: ferredoxin oxidoreductase from the methanogenic archaeon Methanosarcina acetivorans.Structure. 2024 Nov 7;32(11):1963-1972.e3. doi: 10.1016/j.str.2024.08.011. Epub 2024 Sep 11. Structure. 2024. PMID: 39265575 Free PMC article.
-
A structural phylogeny for understanding 2-oxoacid oxidoreductase function.Curr Opin Struct Biol. 2016 Dec;41:54-61. doi: 10.1016/j.sbi.2016.05.011. Epub 2016 Jun 14. Curr Opin Struct Biol. 2016. PMID: 27315560 Free PMC article. Review.
-
X-ray crystallography-based structural elucidation of enzyme-bound intermediates along the 1-deoxy-d-xylulose 5-phosphate synthase reaction coordinate.J Biol Chem. 2019 Aug 16;294(33):12405-12414. doi: 10.1074/jbc.RA119.009321. Epub 2019 Jun 25. J Biol Chem. 2019. PMID: 31239351 Free PMC article.
-
Crystal structures of archaeal 2-oxoacid:ferredoxin oxidoreductases from Sulfolobus tokodaii.Sci Rep. 2016 Sep 13;6:33061. doi: 10.1038/srep33061. Sci Rep. 2016. PMID: 27619895 Free PMC article.
References
-
- Hodgkinson A. Oxalic Acid in Biology and Medicine. Academic; London: 1977.
-
- Hoffman GS, et al. Calcium oxalate microcrystalline-associated arthritis in end-stage renal disease. Ann Intern Med. 1982;97(1):36–42. - PubMed
-
- Marengo SR, Romani AMP. Oxalate in renal stone disease: The terminal metabolite that just won’t go away. Nat Clin Pract Nephrol. 2008;4(7):368–377. - PubMed
-
- Kotsira VP, Clonis YD. Oxalate oxidase from barley roots: Purification to homogeneity and study of some molecular, catalytic, and binding properties. Arch Biochem Biophys. 1997;340(2):239–249. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

