Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans
- PMID: 26712014
- PMCID: PMC4776509
- DOI: 10.1073/pnas.1507110112
Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans
Abstract
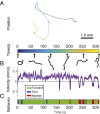
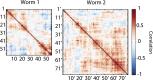
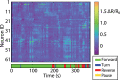
The ability to acquire large-scale recordings of neuronal activity in awake and unrestrained animals is needed to provide new insights into how populations of neurons generate animal behavior. We present an instrument capable of recording intracellular calcium transients from the majority of neurons in the head of a freely behaving Caenorhabditis elegans with cellular resolution while simultaneously recording the animal's position, posture, and locomotion. This instrument provides whole-brain imaging with cellular resolution in an unrestrained and behaving animal. We use spinning-disk confocal microscopy to capture 3D volumetric fluorescent images of neurons expressing the calcium indicator GCaMP6s at 6 head-volumes/s. A suite of three cameras monitor neuronal fluorescence and the animal's position and orientation. Custom software tracks the 3D position of the animal's head in real time and two feedback loops adjust a motorized stage and objective to keep the animal's head within the field of view as the animal roams freely. We observe calcium transients from up to 77 neurons for over 4 min and correlate this activity with the animal's behavior. We characterize noise in the system due to animal motion and show that, across worms, multiple neurons show significant correlations with modes of behavior corresponding to forward, backward, and turning locomotion.
Keywords: C. elegans; behavior; calcium imaging; large-scale recording; microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Comment in
-
Grabbing brain activity on the go.Proc Natl Acad Sci U S A. 2016 Feb 23;113(8):1965-7. doi: 10.1073/pnas.1524219113. Epub 2016 Feb 3. Proc Natl Acad Sci U S A. 2016. PMID: 26842834 Free PMC article. No abstract available.
References
-
- Briggman KL, Abarbanel HDI, Kristan WBJ., Jr Optical imaging of neuronal populations during decision-making. Science. 2005;307(5711):896–901. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

