Protein carbamylation is a hallmark of aging
- PMID: 26712018
- PMCID: PMC4747743
- DOI: 10.1073/pnas.1517096113
Protein carbamylation is a hallmark of aging
Abstract
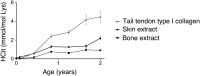
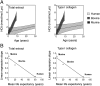
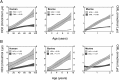
Aging is a progressive process determined by genetic and acquired factors. Among the latter are the chemical reactions referred to as nonenzymatic posttranslational modifications (NEPTMs), such as glycoxidation, which are responsible for protein molecular aging. Carbamylation is a more recently described NEPTM that is caused by the nonenzymatic binding of isocyanate derived from urea dissociation or myeloperoxidase-mediated catabolism of thiocyanate to free amino groups of proteins. This modification is considered an adverse reaction, because it induces alterations of protein and cell properties. It has been shown that carbamylated proteins increase in plasma and tissues during chronic kidney disease and are associated with deleterious clinical outcomes, but nothing is known to date about tissue protein carbamylation during aging. To address this issue, we evaluated homocitrulline rate, the most characteristic carbamylation-derived product (CDP), over time in skin of mammalian species with different life expectancies. Our results show that carbamylation occurs throughout the whole lifespan and leads to tissue accumulation of carbamylated proteins. Because of their remarkably long half-life, matrix proteins, like type I collagen and elastin, are preferential targets. Interestingly, the accumulation rate of CDPs is inversely correlated with longevity, suggesting the occurrence of still unidentified protective mechanisms. In addition, homocitrulline accumulates more intensely than carboxymethyl-lysine, one of the major advanced glycation end products, suggesting the prominent role of carbamylation over glycoxidation reactions in age-related tissue alterations. Thus, protein carbamylation may be considered a hallmark of aging in mammalian species that may significantly contribute in the structural and functional tissue damages encountered during aging.
Keywords: carbamylation; longevity; nonenzymatic posttranslational modifications; skin; tissue aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Comment in
-
Constant molecular aging rates vs. the exponential acceleration of mortality.Proc Natl Acad Sci U S A. 2016 Feb 2;113(5):1121-3. doi: 10.1073/pnas.1524017113. Epub 2016 Jan 20. Proc Natl Acad Sci U S A. 2016. PMID: 26792520 Free PMC article. No abstract available.
Similar articles
-
Carbamylation and glycation compete for collagen molecular aging in vivo.Sci Rep. 2019 Dec 4;9(1):18291. doi: 10.1038/s41598-019-54817-4. Sci Rep. 2019. PMID: 31797985 Free PMC article.
-
Chronic increase of urea leads to carbamylated proteins accumulation in tissues in a mouse model of CKD.PLoS One. 2013 Dec 4;8(12):e82506. doi: 10.1371/journal.pone.0082506. eCollection 2013. PLoS One. 2013. PMID: 24324801 Free PMC article.
-
[Role of protein carbamylation in chronic kidney disease complications].Nephrol Ther. 2015 Jun;11(3):129-34. doi: 10.1016/j.nephro.2014.12.004. Epub 2015 Mar 17. Nephrol Ther. 2015. PMID: 25794932 French.
-
Post-translational modification derived products (PTMDPs): toxins in chronic diseases?Clin Chem Lab Med. 2014 Jan 1;52(1):33-8. doi: 10.1515/cclm-2012-0880. Clin Chem Lab Med. 2014. PMID: 23454717 Review.
-
Carbamylation-derived products: bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis.Clin Chem. 2011 Nov;57(11):1499-505. doi: 10.1373/clinchem.2011.163188. Epub 2011 Jul 18. Clin Chem. 2011. PMID: 21768218 Review.
Cited by
-
Redox-Mediated Carbamylation As a Hapten Model Applied to the Origin of Antibodies to Modified Proteins in Rheumatoid Arthritis.Antioxid Redox Signal. 2022 Mar;36(7-9):389-409. doi: 10.1089/ars.2021.0064. Epub 2021 Jun 4. Antioxid Redox Signal. 2022. PMID: 33906423 Free PMC article. Review.
-
Elevated Plasma Protein Carbonyl Concentration Is Associated with More Abnormal White Matter in People with HIV.Viruses. 2023 Dec 12;15(12):2410. doi: 10.3390/v15122410. Viruses. 2023. PMID: 38140650 Free PMC article.
-
Arrow of Time, Entropy, and Protein Folding: Holistic View on Biochirality.Int J Mol Sci. 2022 Mar 28;23(7):3687. doi: 10.3390/ijms23073687. Int J Mol Sci. 2022. PMID: 35409047 Free PMC article. Review.
-
Constant molecular aging rates vs. the exponential acceleration of mortality.Proc Natl Acad Sci U S A. 2016 Feb 2;113(5):1121-3. doi: 10.1073/pnas.1524017113. Epub 2016 Jan 20. Proc Natl Acad Sci U S A. 2016. PMID: 26792520 Free PMC article. No abstract available.
-
Optimized solubilization of TRIzol-precipitated protein permits Western blotting analysis to maximize data available from brain tissue.J Neurosci Methods. 2017 Mar 15;280:64-76. doi: 10.1016/j.jneumeth.2017.02.002. Epub 2017 Feb 13. J Neurosci Methods. 2017. PMID: 28192129 Free PMC article.
References
-
- Gillery P, Jaisson S. Usefulness of non-enzymatic post-translational modification derived products (PTMDPs) as biomarkers of chronic diseases. J Proteomics. 2013;92:228–238. - PubMed
-
- Li JJ, Surini M, Catsicas S, Kawashima E, Bouras C. Age-dependent accumulation of advanced glycosylation end products in human neurons. Neurobiol Aging. 1995;16(1):69–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

