Protein carbamylation is a hallmark of aging
- PMID: 26712018
- PMCID: PMC4747743
- DOI: 10.1073/pnas.1517096113
Protein carbamylation is a hallmark of aging
Abstract
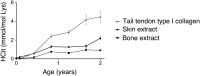
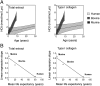
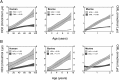
Aging is a progressive process determined by genetic and acquired factors. Among the latter are the chemical reactions referred to as nonenzymatic posttranslational modifications (NEPTMs), such as glycoxidation, which are responsible for protein molecular aging. Carbamylation is a more recently described NEPTM that is caused by the nonenzymatic binding of isocyanate derived from urea dissociation or myeloperoxidase-mediated catabolism of thiocyanate to free amino groups of proteins. This modification is considered an adverse reaction, because it induces alterations of protein and cell properties. It has been shown that carbamylated proteins increase in plasma and tissues during chronic kidney disease and are associated with deleterious clinical outcomes, but nothing is known to date about tissue protein carbamylation during aging. To address this issue, we evaluated homocitrulline rate, the most characteristic carbamylation-derived product (CDP), over time in skin of mammalian species with different life expectancies. Our results show that carbamylation occurs throughout the whole lifespan and leads to tissue accumulation of carbamylated proteins. Because of their remarkably long half-life, matrix proteins, like type I collagen and elastin, are preferential targets. Interestingly, the accumulation rate of CDPs is inversely correlated with longevity, suggesting the occurrence of still unidentified protective mechanisms. In addition, homocitrulline accumulates more intensely than carboxymethyl-lysine, one of the major advanced glycation end products, suggesting the prominent role of carbamylation over glycoxidation reactions in age-related tissue alterations. Thus, protein carbamylation may be considered a hallmark of aging in mammalian species that may significantly contribute in the structural and functional tissue damages encountered during aging.
Keywords: carbamylation; longevity; nonenzymatic posttranslational modifications; skin; tissue aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Comment in
-
Constant molecular aging rates vs. the exponential acceleration of mortality.Proc Natl Acad Sci U S A. 2016 Feb 2;113(5):1121-3. doi: 10.1073/pnas.1524017113. Epub 2016 Jan 20. Proc Natl Acad Sci U S A. 2016. PMID: 26792520 Free PMC article. No abstract available.
References
-
- Gillery P, Jaisson S. Usefulness of non-enzymatic post-translational modification derived products (PTMDPs) as biomarkers of chronic diseases. J Proteomics. 2013;92:228–238. - PubMed
-
- Li JJ, Surini M, Catsicas S, Kawashima E, Bouras C. Age-dependent accumulation of advanced glycosylation end products in human neurons. Neurobiol Aging. 1995;16(1):69–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

