Colorectal delivery and retention of PEG-Amprenavir-Bac7 nanoconjugates--proof of concept for HIV mucosal pre-exposure prophylaxis
- PMID: 26712122
- PMCID: PMC4736156
- DOI: 10.1007/s13346-015-0269-4
Colorectal delivery and retention of PEG-Amprenavir-Bac7 nanoconjugates--proof of concept for HIV mucosal pre-exposure prophylaxis
Abstract
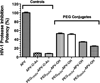
Local delivery of anti-HIV drugs to the colorectal mucosa, a major site of HIV replication, and their retention within mucosal tissue would allow for a reduction in dose administered, reduced dosing frequency and minimal systemic exposure. The current report describes a mucosal pre-exposure prophylaxis (mPrEP) strategy that utilizes nanocarrier conjugates (NC) consisting of poly(ethylene glycol) (PEG), amprenavir (APV), and a cell-penetrating peptide (CPP; namely Bac7, a fragment derived from bactenecin 7). APV-PEG NCs with linear PEGs (2, 5, 10, and 30 kDa) exhibited reduced (52-21%) anti-HIV-1 protease (PR) activity as compared to free APV in an enzyme-based FRET assay. In MT-2 T cells, APV-PEG3.4 kDa-FITC (APF) anti-HIV-1 activity was significantly reduced (160-fold, IC50 = 8064 nM) due to poor cell uptake, whereas it was restored (IC50 = 78.29 nM) and similar to APV (IC50 = 50.29 nM) with the addition of Bac7 to the NC (i.e., APV-PEG3.4 kDa-Bac7, APB). Flow cytometry and confocal microscopy demonstrated Bac7-PEG3.4 kDa-FITC (BPF) uptake was two- and fourfold higher than APF in MT-2 T cells and Caco-2 intestinal epithelial cells, respectively. There was no detectable punctate fluorescence in either cell line suggesting that BPF directly enters the cytosol thus avoiding endosomal entrapment. After colorectal administration in mice, BPF mucosal concentrations were 21-fold higher than APF concentrations. BPF concentrations also remained constant for the 5 days of the study suggesting that (1) the NC's structural characteristics (i.e., the size of the PEG carrier and the presence of a CPP) significantly influenced tissue persistence, and (2) the NCs were probably lodged in the lamina propria since the average rodent colon mucosal cell turnover time is 2-3 days. These encouraging results suggest that Bac7 functionalized NCs delivered locally to the colorectal mucosa may form drug delivery depots that are capable of sustaining colorectal drug concentrations. Although the exact mechanisms for tissue persistence are unclear and will require further study, these results provide proof-of-concept feasibility for mPrEP.
Keywords: Amprenavir; Bactenecin 7; Cell penetrating peptide; Darunavir; HIV; Mucosal delivery; PEG conjugation; Polymeric drug delivery systems; Pre-exposure prophylaxis.
Figures









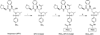



Similar articles
-
Noncovalent PEG Coating of Nanoparticle Drug Carriers Improves the Local Pharmacokinetics of Rectal Anti-HIV Microbicides.ACS Appl Mater Interfaces. 2018 Oct 17;10(41):34942-34953. doi: 10.1021/acsami.8b12214. Epub 2018 Oct 4. ACS Appl Mater Interfaces. 2018. PMID: 30234288
-
Synthesis of poly(ethylene glycol)-based saquinavir prodrug conjugates and assessment of release and anti-HIV-1 bioactivity using a novel protease inhibition assay.Bioconjug Chem. 2004 Nov-Dec;15(6):1322-33. doi: 10.1021/bc0498875. Bioconjug Chem. 2004. PMID: 15546199
-
Genotypic and phenotypic evolution of HIV type-1 protease during in vitro sequential or concomitant combination of atazanavir and amprenavir.Antivir Ther. 2010;15(3):431-6. doi: 10.3851/IMP1543. Antivir Ther. 2010. PMID: 20516562
-
Clinical pharmacology and pharmacokinetics of amprenavir.Ann Pharmacother. 2002 Jan;36(1):102-18. doi: 10.1345/aph.10423. Ann Pharmacother. 2002. PMID: 11816239 Review.
-
Coming therapies: amprenavir.Int J Clin Pract Suppl. 1999 Jun;103:42-4. Int J Clin Pract Suppl. 1999. PMID: 10622044 Review.
Cited by
-
Pharmaceutical Vehicles for Vaginal and Rectal Administration of Anti-HIV Microbicide Nanosystems.Pharmaceutics. 2019 Mar 26;11(3):145. doi: 10.3390/pharmaceutics11030145. Pharmaceutics. 2019. PMID: 30917532 Free PMC article. Review.
-
Triphenylphosphonium Analogs of Short Peptide Related to Bactenecin 7 and Oncocin 112 as Antimicrobial Agents.Pharmaceutics. 2024 Jan 22;16(1):148. doi: 10.3390/pharmaceutics16010148. Pharmaceutics. 2024. PMID: 38276518 Free PMC article.
References
-
- Sheet WAF. [Accessed July 28 2015];Fact sheet N°360. 2015 WHO, http://www.who.int/mediacentre/factsheets/fs360/en/. http://www.who.int/mediacentre/factsheets/fs360/en/
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012;367(5):423–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

