Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab
- PMID: 26712222
- PMCID: PMC5070577
- DOI: 10.1200/JCO.2015.62.5970
Association of Human Papillomavirus and p16 Status With Outcomes in the IMCL-9815 Phase III Registration Trial for Patients With Locoregionally Advanced Oropharyngeal Squamous Cell Carcinoma of the Head and Neck Treated With Radiotherapy With or Without Cetuximab
Abstract
Purpose: We conducted a retrospective evaluation of the IMCL-9815 study to examine the association of human papillomavirus (HPV) and p16 protein expression status with outcomes in patients with oropharyngeal carcinoma (OPC) receiving radiotherapy (RT) plus cetuximab or RT alone.
Patients and methods: In the IMCL-9815 study, patients were randomly allocated to receive RT plus weekly cetuximab or RT alone. A subpopulation of patients with p16-evaluable OPC was retrospectively evaluated on the basis of locoregional control (LRC), overall survival (OS), and progression-free survival (PFS). Evaluable samples from patients with p16-positive OPC were also tested for HPV DNA.
Results: Tumor p16 status was evaluable in 182 patients with OPC enrolled in the IMCL-9815 study; 41% were p16 positive. When treated with RT alone or RT plus cetuximab, p16-positive patients had a longer OS than p16-negative patients (hazard ratio, 0.40; 95% CI, 0.21 to 0.74 and hazard ratio, 0.16; 95% CI, 0.07 to 0.36, respectively). The addition of cetuximab to RT increased LRC, OS, and PFS in both patients with p16-positive OPC and those with p16-negative disease. Interaction tests for LRC, OS, and PFS did not demonstrate any significant interaction between p16 status and treatment effect (P = .087, .085, and .253, respectively). Similar trends were observed when patients with p16-positive/HPV-positive OPC (n = 49) and those with p16-positive/HPV-negative OPC (n = 14) were compared.
Conclusion: p16 status was strongly prognostic for patients with OPC. The data suggest that the addition of cetuximab to RT improved clinical outcomes regardless of p16 or HPV status versus RT alone.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures
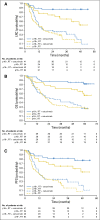
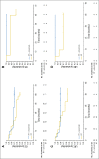
Comment in
-
Cetuximab in Human Papillomavirus-Positive Oropharynx Carcinoma.J Clin Oncol. 2016 Apr 20;34(12):1289-91. doi: 10.1200/JCO.2015.65.1414. Epub 2016 Feb 16. J Clin Oncol. 2016. PMID: 26884569 No abstract available.
References
-
- Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. - PubMed
-
- Zandberg DP, Bhargava R, Badin S, et al. The role of human papillomavirus in nongenital cancers. CA Cancer J Clin. 2013;63:57–81. - PubMed
-
- Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer: Systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. - PubMed
-
- Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

