E-type cyclins modulate telomere integrity in mammalian male meiosis
- PMID: 26712234
- PMCID: PMC4833587
- DOI: 10.1007/s00412-015-0564-3
E-type cyclins modulate telomere integrity in mammalian male meiosis
Abstract
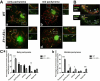
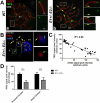
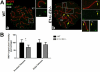
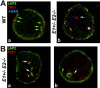
We have shown that E-type cyclins are key regulators of mammalian male meiosis. Depletion of cyclin E2 reduced fertility in male mice due to meiotic defects, involving abnormal pairing and synapsis, unrepaired DNA, and loss of telomere structure. These defects were exacerbated by additional loss of cyclin E1, and complete absence of both E-type cyclins produces a meiotic catastrophe. Here, we investigated the involvement of E-type cyclins in maintaining telomere integrity in male meiosis. Spermatocytes lacking cyclin E2 and one E1 allele (E1+/-E2-/-) displayed a high rate of telomere abnormalities but can progress to pachytene and diplotene stages. We show that their telomeres exhibited an aberrant DNA damage repair response during pachynema and that the shelterin complex proteins TRF2 and RAP2 were significantly decreased in the proximal telomeres. Moreover, the insufficient level of these proteins correlated with an increase of γ-H2AX foci in the affected telomeres and resulted in telomere associations involving TRF1 and telomere detachment in later prophase-I stages. These results suggest that E-type cyclins are key modulators of telomere integrity during meiosis by, at least in part, maintaining the balance of shelterin complex proteins, and uncover a novel role of E-type cyclins in regulating chromosome structure during male meiosis.
Keywords: Cyclin E1; Cyclin E2; E-type cyclins; Meiosis; Meiosis control; Shelterin complex; TRF1; TRF2; Telomere; Telomere integrity.
Figures

Similar articles
-
Mammalian E-type cyclins control chromosome pairing, telomere stability and CDK2 localization in male meiosis.PLoS Genet. 2014 Feb 27;10(2):e1004165. doi: 10.1371/journal.pgen.1004165. eCollection 2014 Feb. PLoS Genet. 2014. PMID: 24586195 Free PMC article.
-
Dual roles of TRF1 in tethering telomeres to the nuclear envelope and protecting them from fusion during meiosis.Cell Death Differ. 2018 Jun;25(6):1174-1188. doi: 10.1038/s41418-017-0037-8. Epub 2018 Jan 8. Cell Death Differ. 2018. PMID: 29311622 Free PMC article.
-
Mammalian meiotic telomeres: protein composition and redistribution in relation to nuclear pores.Mol Biol Cell. 2000 Dec;11(12):4189-203. doi: 10.1091/mbc.11.12.4189. Mol Biol Cell. 2000. PMID: 11102517 Free PMC article.
-
No DDRama at chromosome ends: TRF2 takes centre stage.Trends Biochem Sci. 2015 May;40(5):275-85. doi: 10.1016/j.tibs.2015.03.003. Epub 2015 Apr 3. Trends Biochem Sci. 2015. PMID: 25845889 Review.
-
Post-translational modifications of TRF1 and TRF2 and their roles in telomere maintenance.Mech Ageing Dev. 2012 Jun;133(6):421-34. doi: 10.1016/j.mad.2012.05.002. Epub 2012 May 23. Mech Ageing Dev. 2012. PMID: 22634377 Review.
Cited by
-
Diverse roles for CDK-associated activity during spermatogenesis.FEBS Lett. 2019 Oct;593(20):2925-2949. doi: 10.1002/1873-3468.13627. Epub 2019 Oct 20. FEBS Lett. 2019. PMID: 31566717 Free PMC article. Review.
-
Tethering of Telomeres to the Nuclear Envelope Is Mediated by SUN1-MAJIN and Possibly Promoted by SPDYA-CDK2 During Meiosis.Front Cell Dev Biol. 2020 Sep 4;8:845. doi: 10.3389/fcell.2020.00845. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015044 Free PMC article.
-
Cyclins and CDKs in the regulation of meiosis-specific events.Front Cell Dev Biol. 2022 Nov 29;10:1069064. doi: 10.3389/fcell.2022.1069064. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36523509 Free PMC article. Review.
-
Special issue on "recent advances in meiotic chromosome structure, recombination and segregation".Chromosoma. 2016 Jun;125(2):173-5. doi: 10.1007/s00412-016-0586-5. Epub 2016 Mar 29. Chromosoma. 2016. PMID: 27022980 No abstract available.
-
The Meiosis-Specific Crs1 Cyclin Is Required for Efficient S-Phase Progression and Stable Nuclear Architecture.Int J Mol Sci. 2021 May 22;22(11):5483. doi: 10.3390/ijms22115483. Int J Mol Sci. 2021. PMID: 34067465 Free PMC article.
References
-
- Capanna E, Castiglia R. Chromosomes and speciation in Mus musculus domesticus. Cytogenet Genome Res. 2004;105:375–384. doi:10.1159/000078210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

