Cooperative effects of fibronectin matrix assembly and initial cell-substrate adhesion strength in cellular self-assembly
- PMID: 26712598
- PMCID: PMC4754160
- DOI: 10.1016/j.actbio.2015.12.032
Cooperative effects of fibronectin matrix assembly and initial cell-substrate adhesion strength in cellular self-assembly
Abstract
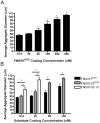
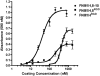
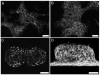
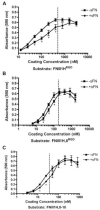
The cell-dependent polymerization of intercellular fibronectin fibrils can stimulate cells to self-assemble into multicellular structures. The local physical cues that support fibronectin-mediated cellular self-assembly are largely unknown. Here, fibronectin matrix analogs were used as synthetic adhesive substrates to model cell-matrix fibronectin fibrils having different integrin-binding specificity, affinity, and/or density. We utilized this model to quantitatively assess the relationship between adhesive forces derived from cell-substrate interactions and the ability of fibronectin fibril assembly to induce cellular self-assembly. Results indicate that the strength of initial, rather than mature, cell-substrate attachments correlates with the ability of substrates to support fibronectin-mediated cellular self-assembly. The cellular response to soluble fibronectin was bimodal and independent of the integrin-binding specificity of the substrate; increasing soluble fibronectin levels above a critical threshold increased aggregate cohesion on permissive substrates. Once aggregates formed, continuous fibronectin polymerization was necessary to maintain cohesion. During self-assembly, soluble fibronectin decreased cell-substrate adhesion strength and induced aggregate cohesion via a Rho-dependent mechanism, suggesting that the balance of contractile forces derived from fibronectin fibrils within cell-cell versus cell-substrate adhesions controls self-assembly and aggregate cohesion. Thus, initial cell-substrate attachment strength may provide a quantitative basis with which to build predictive models of fibronectin-mediated microtissue fabrication on a variety of substrates.
Statement of significance: Cellular self-assembly is a process by which cells and extracellular matrix (ECM) proteins spontaneously organize into three-dimensional (3D) tissues in the absence of external forces. Cellular self-assembly can be initiated in vitro, and represents a potential tool for tissue engineers to organize cells into modular building blocks for artificial tissue fabrication. Fibronectin is an ECM protein that plays a key role in tissue formation during embryonic development. Additionally, the cell-mediated process of converting soluble fibronectin into insoluble, ECM-associated fibrils has been shown to initiate cellular self-assembly in vitro. In this study, we examine the relationship between the strength of cell-substrate adhesions and the ability of fibronectin fibril assembly to induce cellular self-assembly. Our results indicate that substrate composition and density play cooperative roles with cell-mediated fibronectin matrix assembly to control the transition of cells from 2D monolayers into 3D multicellular aggregates. Results of this study provide a quantitative approach to build predictive models of cellular self-assembly, as well as a simple cell-culture platform to produce biomimetic units for modular tissue engineering.
Keywords: Biomimetic material; Cell adhesion; Extracellular matrix; Fibronectin; Self-assembly.
Copyright © 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
Regional fibronectin and collagen fibril co-assembly directs cell proliferation and microtissue morphology.PLoS One. 2013 Oct 8;8(10):e77316. doi: 10.1371/journal.pone.0077316. eCollection 2013. PLoS One. 2013. PMID: 24116223 Free PMC article.
-
Extracellular matrix fibronectin stimulates the self-assembly of microtissues on native collagen gels.Tissue Eng Part A. 2010 Dec;16(12):3805-19. doi: 10.1089/ten.TEA.2010.0316. Epub 2010 Sep 6. Tissue Eng Part A. 2010. PMID: 20673131 Free PMC article.
-
Recombinant fibronectin matrix mimetics specify integrin adhesion and extracellular matrix assembly.Tissue Eng Part A. 2013 Feb;19(3-4):558-70. doi: 10.1089/ten.TEA.2012.0257. Epub 2012 Nov 1. Tissue Eng Part A. 2013. PMID: 23020251 Free PMC article.
-
Assembly of fibronectin extracellular matrix.Annu Rev Cell Dev Biol. 2010;26:397-419. doi: 10.1146/annurev-cellbio-100109-104020. Annu Rev Cell Dev Biol. 2010. PMID: 20690820 Free PMC article. Review.
-
The ins and outs of fibronectin matrix assembly.J Cell Sci. 2003 Aug 15;116(Pt 16):3269-76. doi: 10.1242/jcs.00670. J Cell Sci. 2003. PMID: 12857786 Review.
Cited by
-
Depleting RhoA/Stress Fiber-Organized Fibronectin Matrices on Tumor Cells Non-Autonomously Aggravates Fibroblast-Driven Tumor Cell Growth.Int J Mol Sci. 2020 Nov 4;21(21):8272. doi: 10.3390/ijms21218272. Int J Mol Sci. 2020. PMID: 33158289 Free PMC article.
-
Model of collective detachment in high-grade serous ovarian cancer demonstrates that tumor spheroids produce ECM to support metastatic processes.APL Bioeng. 2023 Feb 28;7(1):016111. doi: 10.1063/5.0132254. eCollection 2023 Mar. APL Bioeng. 2023. PMID: 36875739 Free PMC article.
-
Extracellular matrix fibronectin mediates an endothelial cell response to shear stress via the heparin-binding, matricryptic RWRPK sequence of FNIII1H.Am J Physiol Heart Circ Physiol. 2016 Oct 1;311(4):H1063-H1071. doi: 10.1152/ajpheart.00126.2016. Epub 2016 Aug 12. Am J Physiol Heart Circ Physiol. 2016. PMID: 27521419 Free PMC article.
-
Clustering of integrin α5 at the lateral membrane restores epithelial polarity in invasive colorectal cancer cells.Mol Biol Cell. 2017 May 15;28(10):1288-1300. doi: 10.1091/mbc.E16-12-0852. Epub 2017 Mar 29. Mol Biol Cell. 2017. PMID: 28356422 Free PMC article.
-
Epithelial multicellular clustering enabled by polarized macrophages on soft matrices.FASEB J. 2023 Aug;37(8):e23059. doi: 10.1096/fj.202300120RR. FASEB J. 2023. PMID: 37389911 Free PMC article.
References
-
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. BioEssays. 2000;22:138–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

