Proton magnetic resonance spectroscopy in oncology: the fingerprints of cancer?
- PMID: 26712681
- PMCID: PMC4712903
- DOI: 10.5152/dir.2015.15009
Proton magnetic resonance spectroscopy in oncology: the fingerprints of cancer?
Abstract
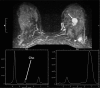
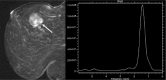
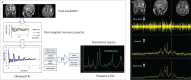
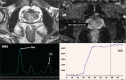
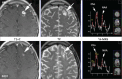
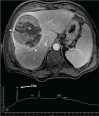
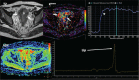
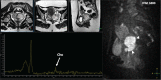
Abnormal metabolism is a key tumor hallmark. Proton magnetic resonance spectroscopy (1H-MRS) allows measurement of metabolite concentration that can be utilized to characterize tumor metabolic changes. 1H-MRS measurements of specific metabolites have been implemented in the clinic. This article performs a systematic review of image acquisition and interpretation of 1H-MRS for cancer evaluation, evaluates its strengths and limitations, and correlates metabolite peaks at 1H-MRS with diagnostic and prognostic parameters of cancer in different tumor types.
Figures
Similar articles
-
Deciphering the role of glial cell-specific metabolites as biomarkers in early cervical myelopathy-insights from in vivo MRS study.Spine J. 2025 May 24:S1529-9430(25)00272-4. doi: 10.1016/j.spinee.2025.05.031. Online ahead of print. Spine J. 2025. PMID: 40418991
-
MRI software and cognitive fusion biopsies in people with suspected prostate cancer: a systematic review, network meta-analysis and cost-effectiveness analysis.Health Technol Assess. 2024 Oct;28(61):1-310. doi: 10.3310/PLFG4210. Health Technol Assess. 2024. PMID: 39367754 Free PMC article.
-
Neurochemical changes in patients with chronic low back pain detected by proton magnetic resonance spectroscopy: A systematic review.Neuroimage Clin. 2016 Nov 24;13:33-38. doi: 10.1016/j.nicl.2016.11.006. eCollection 2017. Neuroimage Clin. 2016. PMID: 27920977 Free PMC article.
-
Positron emission tomography (PET) and magnetic resonance imaging (MRI) for the assessment of axillary lymph node metastases in early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2011 Jan;15(4):iii-iv, 1-134. doi: 10.3310/hta15040. Health Technol Assess. 2011. PMID: 21276372 Free PMC article.
-
Structural neuroimaging in psychosis: a systematic review and economic evaluation.Health Technol Assess. 2008 May;12(18):iii-iv, ix-163. doi: 10.3310/hta12180. Health Technol Assess. 2008. PMID: 18462577
Cited by
-
Small molecule metabolites: discovery of biomarkers and therapeutic targets.Signal Transduct Target Ther. 2023 Mar 20;8(1):132. doi: 10.1038/s41392-023-01399-3. Signal Transduct Target Ther. 2023. PMID: 36941259 Free PMC article. Review.
-
Imaging biomarkers from multiparametric magnetic resonance imaging are associated with survival outcomes in patients with brain metastases from breast cancer.Eur Radiol. 2018 Nov;28(11):4860-4870. doi: 10.1007/s00330-018-5448-5. Epub 2018 May 16. Eur Radiol. 2018. PMID: 29770848
-
1H-MRS application in the evaluation of response to photo-thermal therapy using iron oxide-gold core-shell nanoparticles, an in vivo study.Photochem Photobiol Sci. 2021 Feb;20(2):245-254. doi: 10.1007/s43630-021-00012-2. Epub 2021 Feb 9. Photochem Photobiol Sci. 2021. PMID: 33721249
-
DKI and 1H-MRS in angiogenesis evaluation of soft tissue sarcomas: a prospective clinical study based on MRI-pathology control method.BMC Med Imaging. 2024 Dec 18;24(1):340. doi: 10.1186/s12880-024-01526-8. BMC Med Imaging. 2024. PMID: 39695437 Free PMC article.
-
The Use of Magnetic Resonance Imaging in Radiation Therapy Treatment Simulation and Planning.J Magn Reson Imaging. 2024 Nov;60(5):1786-1805. doi: 10.1002/jmri.29246. Epub 2024 Jan 24. J Magn Reson Imaging. 2024. PMID: 38265188 Review.
References
-
- Hajek M, Dezortova M. Introduction to clinical in vivo MR spectroscopy. Eur J Radiol. 2008;67:185–193. http://dx.doi.org/10.1016/j.ejrad.2008.03.002. - DOI - PubMed
-
- Glunde K, Bhujwalla ZM. Metabolic tumor imaging using magnetic resonance spectroscopy. Semin Oncol. 2011;38:26–41. http://dx.doi.org/10.1053/j.seminoncol.2010.11.001. - DOI - PMC - PubMed
-
- Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11:835–848. http://dx.doi.org/10.1038/nrc3162. - DOI - PMC - PubMed
-
- Kwock L, Smith JK, Castillo M, et al. Clinical role of proton magnetic resonance spectroscopy in oncology: brain, breast, and prostate cancer. Lancet Oncol. 2006;7:859–868. http://dx.doi.org/10.1016/S1470-2045(06)70905-6. - DOI - PubMed
-
- Jansen JF, Carlson DL, Lu Y, et al. Correlation of a prior DCE-MRI and (1)H-MRS data with molecular markers in neck nodal metastases: Initial analysis. Oral Oncol. 2012;48:717–722. http://dx.doi.org/10.1016/j.oraloncology.2012.02.001. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

