Benz[c,d]indolium-containing Monomethine Cyanine Dyes: Synthesis and Photophysical Properties
- PMID: 26712725
- PMCID: PMC6274575
- DOI: 10.3390/molecules21010023
Benz[c,d]indolium-containing Monomethine Cyanine Dyes: Synthesis and Photophysical Properties
Abstract
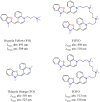
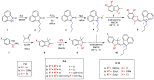
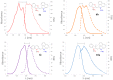
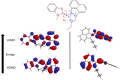
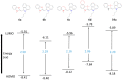
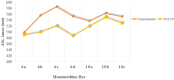
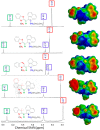
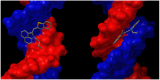
Asymmetric monomethine cyanines have been extensively used as probes for nucleic acids among other biological systems. Herein we report the synthesis of seven monomethine cyanine dyes that have been successfully prepared with various heterocyclic moieties such as quinoline, benzoxazole, benzothiazole, dimethyl indole, and benz[e]indole adjoining benz[c,d]indol-1-ium, which was found to directly influence their optical and energy profiles. In this study the optical properties vs. structural changes were investigated using nuclear magnetic resonance and computational approaches. The twisted conformation unique to monomethine cyanines was exploited in DNA binding studies where the newly designed sensor displayed an increase in fluorescence when bound in the DNA grooves compared to the unbound form.
Keywords: DFT calculations; DNA grooves; cyanine dye; optical properties; synthesis; unsymmetrical.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

