Peptide KSL-W-Loaded Mucoadhesive Liquid Crystalline Vehicle as an Alternative Treatment for Multispecies Oral Biofilm
- PMID: 26712726
- PMCID: PMC6273598
- DOI: 10.3390/molecules21010037
Peptide KSL-W-Loaded Mucoadhesive Liquid Crystalline Vehicle as an Alternative Treatment for Multispecies Oral Biofilm
Abstract
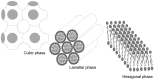
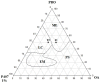
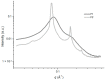
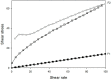
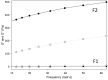
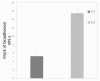
Decapeptide KSL-W shows antibacterial activities and can be used in the oral cavity, however, it is easily degraded in aqueous solution and eliminated. Therefore, we aimed to develop liquid crystalline systems (F1 and F2) for KSL-W buccal administration to treat multispecies oral biofilms. The systems were prepared with oleic acid, polyoxypropylene (5) polyoxyethylene (20) cetyl alcohol (PPG-5-CETETH-20), and a 1% poloxamer 407 dispersion as the oil phase (OP), surfactant (S), and aqueous phase (AP), respectively. We characterized them using polarized light microscopy (PLM), small-angle X-ray scattering (SAXS), rheology, and in vitro bioadhesion, and performed in vitro biological analysis. PLM showed isotropy (F1) or anisotropy with lamellar mesophases (F2), confirmed by peak ratio quantification using SAXS. Rheological tests demonstrated that F1 exhibited Newtonian behavior but not F2, which showed a structured AP concentration-dependent system. Bioadhesion studies revealed an AP concentration-dependent increase in the system's bioadhesiveness (F2 = 15.50 ± 1.00 mN·s) to bovine teeth blocks. Antimicrobial testing revealed 100% inhibition of multispecies oral biofilm growth after KSL-W administration, which was incorporated in the F2 aqueous phase at a concentration of 1 mg/mL. Our results suggest that this system could serve as a potential vehicle for buccal administration of antibiofilm peptides.
Keywords: KSL-W; antimicrobial peptide; biofilm; liquid crystalline systems; oral cavity.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Sixou M., Diouf A., Alvares D. Biofilm buccal et pathologies buccodentaires. Antibiotiques. 2007;9:181–188. doi: 10.1016/S1294-5501(07)91377-1. - DOI
-
- Taweechaisupapong S., Pinsuwan W., Suwannarong W., Kukhetpitakwong R., Luengpailin S. Effects of Streblus asper leaf extract on the biofilm formation of subgingival pathogens. S. Afr. J. Bot. 2014;94:1–5. doi: 10.1016/j.sajb.2014.05.005. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

