Resistance to Recombinant Human Erythropoietin Therapy in a Rat Model of Chronic Kidney Disease Associated Anemia
- PMID: 26712750
- PMCID: PMC4730274
- DOI: 10.3390/ijms17010028
Resistance to Recombinant Human Erythropoietin Therapy in a Rat Model of Chronic Kidney Disease Associated Anemia
Abstract
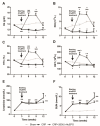
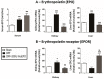
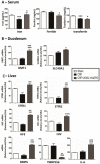
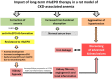
This study aimed to elucidate the mechanisms explaining the persistence of anemia and resistance to recombinant human erythropoietin (rHuEPO) therapy in a rat model of chronic kidney disease (CKD)-associated anemia with formation of anti-rHuEPO antibodies. The remnant kidney rat model of CKD induced by 5/6 nephrectomy was used to test a long-term (nine weeks) high dose of rHuEPO (200 UI/kg bw/week) treatment. Hematological and biochemical parameters were evaluated as well as serum and tissue (kidney, liver and/or duodenum) protein and/or gene expression of mediators of erythropoiesis, iron metabolism and tissue hypoxia, inflammation, and fibrosis. Long-term treatment with a high rHuEPO dose is associated with development of resistance to therapy as a result of antibodies formation. In this condition, serum EPO levels are not deficient and iron availability is recovered by increased duodenal absorption. However, erythropoiesis is not stimulated, and the resistance to endogenous EPO effect and to rHuEPO therapy results from the development of a hypoxic, inflammatory and fibrotic milieu in the kidney tissue. This study provides new insights that could be important to ameliorate the current therapeutic strategies used to treat patients with CKD-associated anemia, in particular those that become resistant to rHuEPO therapy.
Keywords: anemia; chronic kidney disease; erythropoiesis; inflammation and fibrosis; iron metabolism; kidney hypoxia; remnant kidney rat model; resistance to rHuEPO therapy.
Figures







Similar articles
-
Pathological and molecular mechanisms underlying resistance to recombinant human erythropoietin therapy in the remnant kidney rat model of chronic kidney disease associated anemia.Biochimie. 2016 Jun;125:150-62. doi: 10.1016/j.biochi.2016.03.012. Epub 2016 Mar 31. Biochimie. 2016. PMID: 27039028
-
Iron-hepcidin dysmetabolism, anemia and renal hypoxia, inflammation and fibrosis in the remnant kidney rat model.PLoS One. 2015 Apr 13;10(4):e0124048. doi: 10.1371/journal.pone.0124048. eCollection 2015. PLoS One. 2015. PMID: 25867633 Free PMC article.
-
Liver iron is a major regulator of hepcidin gene expression via BMP/SMAD pathway in a rat model of chronic renal failure under treatment with high rHuEPO doses.Biofactors. 2016 May;42(3):296-306. doi: 10.1002/biof.1275. Epub 2016 Mar 18. Biofactors. 2016. PMID: 26990350
-
Erythropoietin hyporesponsiveness: from iron deficiency to iron overload.Kidney Int Suppl. 1999 Mar;69:S107-18. Kidney Int Suppl. 1999. PMID: 10084294 Review.
-
The role of iron in erythropoiesis in the absence and presence of erythropoietin therapy.Nephrol Dial Transplant. 2002;17 Suppl 5:14-8. doi: 10.1093/ndt/17.suppl_5.14. Nephrol Dial Transplant. 2002. PMID: 12091601 Review.
Cited by
-
A Chinese herbal decoction, Jian-Pi-Yi-Shen, regulates the expressions of erythropoietin and pro-inflammatory cytokines in cultured cells.BMC Complement Altern Med. 2018 Apr 3;18(1):119. doi: 10.1186/s12906-018-2146-4. BMC Complement Altern Med. 2018. PMID: 29615029 Free PMC article.
-
Alkaloid fraction of Mirabilis jalapa Linn. flowers has low cytotoxicity and increases iron absorption through Erythropoietin-Matriptase-2-Hepcidin pathway in iron deficiency Hepatocarcinoma cell model.Saudi J Biol Sci. 2023 Jan;30(1):103508. doi: 10.1016/j.sjbs.2022.103508. Epub 2022 Nov 18. Saudi J Biol Sci. 2023. PMID: 36471797 Free PMC article.
-
Advances in Chronic Kidney Disease.Int J Mol Sci. 2016 Aug 11;17(8):1314. doi: 10.3390/ijms17081314. Int J Mol Sci. 2016. PMID: 27529223 Free PMC article.
-
Alternative Erythropoietin Receptors in the Nervous System.J Clin Med. 2018 Feb 2;7(2):24. doi: 10.3390/jcm7020024. J Clin Med. 2018. PMID: 29393890 Free PMC article. Review.
-
Change in iron metabolism in rats after renal ischemia/reperfusion injury.PLoS One. 2017 Apr 20;12(4):e0175945. doi: 10.1371/journal.pone.0175945. eCollection 2017. PLoS One. 2017. PMID: 28426710 Free PMC article.
References
-
- Stevens L.A., Li S., Wang C., Huang C., Becker B.N., Bomback A.S., Brown W.W., Burrows N.R., Jurkovitz C.T., McFarlane S.I., et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the Kidney Early Evaluation Program (KEEP) Am. J. Kidney Dis. 2010;55:S23–S33. doi: 10.1053/j.ajkd.2009.09.035. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

