MicroRNA Regulation of Human Breast Cancer Stem Cells
- PMID: 26712794
- PMCID: PMC4730127
- DOI: 10.3390/jcm5010002
MicroRNA Regulation of Human Breast Cancer Stem Cells
Abstract
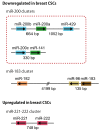
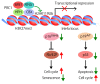
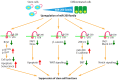
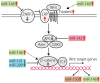
MicroRNAs (miRNAs) are involved in virtually all biological processes, including stem cell maintenance, differentiation, and development. The dysregulation of miRNAs is associated with many human diseases including cancer. We have identified a set of miRNAs differentially expressed between human breast cancer stem cells (CSCs) and non-tumorigenic cancer cells. In addition, these miRNAs are similarly upregulated or downregulated in normal mammary stem/progenitor cells. In this review, we mainly describe the miRNAs that are dysregulated in human breast CSCs directly isolated from clinical specimens. The miRNAs and their clusters, such as the miR-200 clusters, miR-183 cluster, miR-221-222 cluster, let-7, miR-142 and miR-214, target the genes and pathways important for stem cell maintenance, such as the self-renewal gene BMI1, apoptosis, Wnt signaling, Notch signaling, and epithelial-to-mesenchymal transition. In addition, the current evidence shows that metastatic breast CSCs acquire a phenotype that is different from the CSCs in a primary site. Thus, clarifying the miRNA regulation of the metastatic breast CSCs will further advance our understanding of the roles of human breast CSCs in tumor progression.
Keywords: Bmi1; Wnt signaling; cancer stem cells; epithelial-to-mesenchymal transition (EMT); metastasis; microRNA.
Figures
Similar articles
-
microRNA regulation of human pancreatic cancer stem cells.Stem Cell Investig. 2017 Jan 21;4:5. doi: 10.21037/sci.2017.01.01. eCollection 2017. Stem Cell Investig. 2017. PMID: 28217707 Free PMC article. Review.
-
MicroRNA-regulated gene networks during mammary cell differentiation are associated with breast cancer.Carcinogenesis. 2012 Aug;33(8):1502-11. doi: 10.1093/carcin/bgs161. Epub 2012 May 4. Carcinogenesis. 2012. PMID: 22562546
-
MicroRNA expression profile of colon cancer stem-like cells in HT29 adenocarcinoma cell line.Biochem Biophys Res Commun. 2011 Jan 7;404(1):273-8. doi: 10.1016/j.bbrc.2010.11.106. Epub 2010 Dec 2. Biochem Biophys Res Commun. 2011. PMID: 21130073
-
Distinctive role of dysregulated miRNAs in chordoma cancer stem-like cell maintenance.Exp Cell Res. 2019 Jul 1;380(1):9-19. doi: 10.1016/j.yexcr.2019.03.039. Epub 2019 Apr 2. Exp Cell Res. 2019. PMID: 30951707
-
Embryonic stem cell microRNAs: defining factors in induced pluripotent (iPS) and cancer (CSC) stem cells?Curr Stem Cell Res Ther. 2009 Sep;4(3):168-77. doi: 10.2174/157488809789057400. Curr Stem Cell Res Ther. 2009. PMID: 19492978 Review.
Cited by
-
Effects of miRNAs on functions of breast cancer stem cells and treatment of breast cancer.Onco Targets Ther. 2018 Jul 24;11:4263-4270. doi: 10.2147/OTT.S165156. eCollection 2018. Onco Targets Ther. 2018. PMID: 30100733 Free PMC article. Review.
-
MicroRNA-183 inhibits A375 human melanoma cell migration and invasion by targeting Ezrin and MMP-9.Oncol Lett. 2019 Jan;17(1):548-554. doi: 10.3892/ol.2018.9603. Epub 2018 Oct 22. Oncol Lett. 2019. PMID: 30655800 Free PMC article.
-
miR-376c-3p modulates the properties of breast cancer stem cells by targeting RAB2A.Exp Ther Med. 2020 Nov;20(5):68. doi: 10.3892/etm.2020.9196. Epub 2020 Sep 9. Exp Ther Med. 2020. PMID: 32963598 Free PMC article.
-
Transgenic overexpression of the miR-200b/200a/429 cluster inhibits mammary tumor initiation.Transl Oncol. 2021 Dec;14(12):101228. doi: 10.1016/j.tranon.2021.101228. Epub 2021 Sep 22. Transl Oncol. 2021. PMID: 34562686 Free PMC article.
-
microRNA regulation of human pancreatic cancer stem cells.Stem Cell Investig. 2017 Jan 21;4:5. doi: 10.21037/sci.2017.01.01. eCollection 2017. Stem Cell Investig. 2017. PMID: 28217707 Free PMC article. Review.
References
-
- Cohnheim J. Congenitales, quergestreiftes muskelsarkom der nieren. Arch. Pathol. Anatom. Physiol. Klin. Med. 1875;65:64–69. doi: 10.1007/BF01978936. - DOI
-
- Fialkow P.J. Human tumors studied with genetic markers. Birth Defects Orig. Artic. Ser. 1976;12:123–132. - PubMed
-
- Fialkow P.J. Stem cell origin of human myeloid blood cell neoplasms. Verh. Dtsch. Ges. Pathol. 1990;74:43–47. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

