High membrane permeability for melatonin
- PMID: 26712850
- PMCID: PMC4692493
- DOI: 10.1085/jgp.201511526
High membrane permeability for melatonin
Abstract
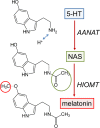
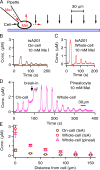
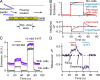
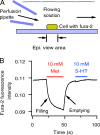
The pineal gland, an endocrine organ in the brain, synthesizes and secretes the circulating night hormone melatonin throughout the night. The literature states that this hormone is secreted by simple diffusion across the pinealocyte plasma membrane, but a direct quantitative measurement of membrane permeability has not been made. Experiments were designed to compare the cell membrane permeability to three indoleamines: melatonin and its precursors N-acetylserotonin (NAS) and serotonin (5-HT). The three experimental approaches were (1) to measure the concentration of effluxing indoleamines amperometrically in the bath while cells were being dialyzed internally by a patch pipette, (2) to measure the rise of intracellular indoleamine fluorescence as the compound was perfused in the bath, and (3) to measure the rate of quenching of intracellular fura-2 dye fluorescence as indoleamines were perfused in the bath. These measures showed that permeabilities of melatonin and NAS are high (both are uncharged molecules), whereas that for 5-HT (mostly charged) is much lower. Comparisons were made with predictions of solubility-diffusion theory and compounds of known permeability, and a diffusion model was made to simulate all of the measurements. In short, extracellular melatonin equilibrates with the cytoplasm in 3.5 s, has a membrane permeability of ∼1.7 µm/s, and could not be retained in secretory vesicles. Thus, it and NAS will be "secreted" from pineal cells by membrane diffusion. Circumstances are suggested when 5-HT and possibly catecholamines may also appear in the extracellular space passively by membrane diffusion.
© 2016 Yu et al.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

