Permuting the PGF Signature Motif Blocks both Archaeosortase-Dependent C-Terminal Cleavage and Prenyl Lipid Attachment for the Haloferax volcanii S-Layer Glycoprotein
- PMID: 26712937
- PMCID: PMC4810604
- DOI: 10.1128/JB.00849-15
Permuting the PGF Signature Motif Blocks both Archaeosortase-Dependent C-Terminal Cleavage and Prenyl Lipid Attachment for the Haloferax volcanii S-Layer Glycoprotein
Abstract
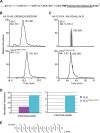
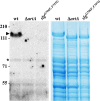
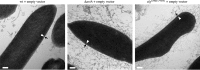
For years, the S-layer glycoprotein (SLG), the sole component of many archaeal cell walls, was thought to be anchored to the cell surface by a C-terminal transmembrane segment. Recently, however, we demonstrated that the Haloferax volcanii SLG C terminus is removed by an archaeosortase (ArtA), a novel peptidase. SLG, which was previously shown to be lipid modified, contains a C-terminal tripartite structure, including a highly conserved proline-glycine-phenylalanine (PGF) motif. Here, we demonstrate that ArtA does not process an SLG variant where the PGF motif is replaced with a PFG motif (slg(G796F,F797G)). Furthermore, using radiolabeling, we show that SLG lipid modification requires the PGF motif and is ArtA dependent, lending confirmation to the use of a novel C-terminal lipid-mediated protein-anchoring mechanism by prokaryotes. Similar to the case for the ΔartA strain, the growth, cellular morphology, and cell wall of the slg(G796F,F797G) strain, in which modifications of additional H. volcanii ArtA substrates should not be altered, are adversely affected, demonstrating the importance of these posttranslational SLG modifications. Our data suggest that ArtA is either directly or indirectly involved in a novel proteolysis-coupled, covalent lipid-mediated anchoring mechanism. Given that archaeosortase homologs are encoded by a broad range of prokaryotes, it is likely that this anchoring mechanism is widely conserved.
Importance: Prokaryotic proteins bound to cell surfaces through intercalation, covalent attachment, or protein-protein interactions play critical roles in essential cellular processes. Unfortunately, the molecular mechanisms that anchor proteins to archaeal cell surfaces remain poorly characterized. Here, using the archaeon H. volcanii as a model system, we report the first in vivo studies of a novel protein-anchoring pathway involving lipid modification of a peptidase-processed C terminus. Our findings not only yield important insights into poorly understood aspects of archaeal biology but also have important implications for key bacterial species, including those of the human microbiome. Additionally, insights may facilitate industrial applications, given that photosynthetic cyanobacteria encode uncharacterized homologs of this evolutionarily conserved enzyme, or may spur development of unique drug delivery systems.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

