Iron Acquisition in Mycobacterium avium subsp. paratuberculosis
- PMID: 26712939
- PMCID: PMC4810606
- DOI: 10.1128/JB.00922-15
Iron Acquisition in Mycobacterium avium subsp. paratuberculosis
Abstract
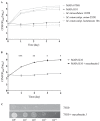
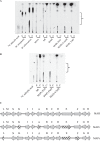
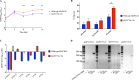
Mycobacterium avium subsp. paratuberculosis is a host-adapted pathogen that evolved from the environmental bacterium M. avium subsp. hominissuis through gene loss and gene acquisition. Growth of M. avium subsp. paratuberculosis in the laboratory is enhanced by supplementation of the media with the iron-binding siderophore mycobactin J. Here we examined the production of mycobactins by related organisms and searched for an alternative iron uptake system in M. avium subsp. paratuberculosis. Through thin-layer chromatography and radiolabeled iron-uptake studies, we showed that M. avium subsp. paratuberculosis is impaired for both mycobactin synthesis and iron acquisition. Consistent with these observations, we identified several mutations, including deletions, in M. avium subsp. paratuberculosis genes coding for mycobactin synthesis. Using a transposon-mediated mutagenesis screen conditional on growth without myobactin, we identified a potential mycobactin-independent iron uptake system on a M. avium subsp. paratuberculosis-specific genomic island, LSP(P)15. We obtained a transposon (Tn) mutant with a disruption in the LSP(P)15 gene MAP3776c for targeted study. The mutant manifests increased iron uptake as well as intracellular iron content, with genes downstream of the transposon insertion (MAP3775c to MAP3772c [MAP3775-2c]) upregulated as the result of a polar effect. As an independent confirmation, we observed the same iron uptake phenotypes by overexpressing MAP3775-2c in wild-type M. avium subsp. paratuberculosis. These data indicate that the horizontally acquired LSP(P)15 genes contribute to iron acquisition by M. avium subsp. paratuberculosis, potentially allowing the subsequent loss of siderophore production by this pathogen.
Importance: Many microbes are able to scavenge iron from their surroundings by producing iron-chelating siderophores. One exception is Mycobacterium avium subsp. paratuberculosis, a fastidious, slow-growing animal pathogen whose growth needs to be supported by exogenous mycobacterial siderophore (mycobactin) in the laboratory. Data presented here demonstrate that, compared to other closely related M. avium subspecies, mycobactin production and iron uptake are different in M. avium subsp. paratuberculosis, and these phenotypes may be caused by numerous deletions in its mycobactin biosynthesis pathway. Using a genomic approach, supplemented by targeted genetic and biochemical studies, we identified that LSP(P)15, a horizontally acquired genomic island, may encode an alternative iron uptake system. These findings shed light on the potential physiological consequence of horizontal gene transfer in M. avium subsp. paratuberculosis evolution.
Copyright © 2016 Wang et al.
Figures

Similar articles
-
Inability to detect mycobactin in mycobacteria-infected tissues suggests an alternative iron acquisition mechanism by mycobacteria in vivo.Microb Pathog. 1993 Mar;14(3):229-38. doi: 10.1006/mpat.1993.1022. Microb Pathog. 1993. PMID: 8321124
-
Iron-binding compounds of Mycobacterium avium, M. intracellulare, M. scrofulaceum, and mycobactin-dependent M. paratuberculosis and M. avium.J Bacteriol. 1983 Mar;153(3):1138-46. doi: 10.1128/jb.153.3.1138-1146.1983. J Bacteriol. 1983. PMID: 6826517 Free PMC article.
-
Extensive genomic polymorphism within Mycobacterium avium.J Bacteriol. 2004 Sep;186(18):6332-4. doi: 10.1128/JB.186.18.6332-6334.2004. J Bacteriol. 2004. PMID: 15342607 Free PMC article.
-
Application of the genome sequence to address concerns that Mycobacterium avium subspecies paratuberculosis might be a foodborne pathogen.Foodborne Pathog Dis. 2004 Spring;1(1):3-15. doi: 10.1089/153531404772914419. Foodborne Pathog Dis. 2004. PMID: 15992257 Review.
-
Genetic diversity and phylogeny of Mycobacterium avium.Infect Genet Evol. 2014 Jan;21:375-83. doi: 10.1016/j.meegid.2013.12.007. Epub 2013 Dec 15. Infect Genet Evol. 2014. PMID: 24345519 Review.
Cited by
-
The many lives of nontuberculous mycobacteria.J Bacteriol. 2018 Feb 26;200(11):e00739-17. doi: 10.1128/JB.00739-17. Online ahead of print. J Bacteriol. 2018. PMID: 29483164 Free PMC article.
-
Identification and Characterization of Mycobacterium smegmatis and Mycobacterium avium subsp. paratuberculosis Zinc Transporters.J Bacteriol. 2021 May 1;203(9):e00049-21. doi: 10.1128/JB.00049-21. Epub 2021 Mar 15. J Bacteriol. 2021. PMID: 33722846 Free PMC article.
-
Paratuberculosis: A Potential Zoonosis and a Neglected Disease in Africa.Microorganisms. 2020 Jul 5;8(7):1007. doi: 10.3390/microorganisms8071007. Microorganisms. 2020. PMID: 32635652 Free PMC article. Review.
-
Characterization of the bovine salivary gland transcriptome associated with Mycobacterium avium subsp. paratuberculosis experimental challenge.BMC Genomics. 2019 Jun 13;20(1):491. doi: 10.1186/s12864-019-5845-4. BMC Genomics. 2019. PMID: 31195975 Free PMC article.
-
Elucidating the Regulon of a Fur-like Protein in Mycobacterium avium subsp. paratuberculosis (MAP).Front Microbiol. 2020 Apr 23;11:598. doi: 10.3389/fmicb.2020.00598. eCollection 2020. Front Microbiol. 2020. PMID: 32390963 Free PMC article.
References
-
- McFadyean J. 1906. A new disease of cattle (Johne's disease). Annual report for 1906 of the principal of the Royal Veterinary College. J Roy Agric Soc Engl 6:230–241.
-
- Twort FW, Ingram GLY. 1912. A method for isolating and cultivating the Mycobacterium enteritidis chronicae pseudotuberculosae bovis, Johne, and some experiments on the preparation of a diagnostic vaccine for pseudo-tuberculous enteritis of bovines. Proc R Soc Lond B Biol Sci 84:517–542.
-
- Ratledge C. 2013. A history of iron metabolism in the Mycobacteria, p 3–39. In Byers BR. (ed), Iron acquisition by the genus Mycobacterium. Springer International Publishing, Cham, Switzerland.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

