Potent Triazole Bisphosphonate Inhibitor of Geranylgeranyl Diphosphate Synthase
- PMID: 26713103
- PMCID: PMC4677368
- DOI: 10.1021/acsmedchemlett.5b00334
Potent Triazole Bisphosphonate Inhibitor of Geranylgeranyl Diphosphate Synthase
Abstract
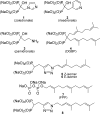
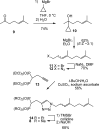
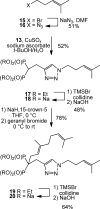
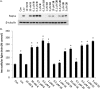
Studies of triazole bisphosphonates have resulted in identification of a potent inhibitor of geranylgeranyl diphosphate synthase (IC50 = 45 nM) with very good selectivity for this enzyme over farnesyl diphosphate synthase (IC50 = 28 μM). This compound also potently disrupts geranylgeranylation and induces cytotoxicity in human myeloma cells at submicromolar levels, suggesting that it may serve as a lead compound for treatment of malignancies characterized by excessive protein secretion.
Keywords: GGDPS; Geranylgeranyl diphosphate synthase; bisphosphonate; farnesyl diphosphate synthase; myeloma.
Conflict of interest statement
The authors declare the following competing financial interest(s): D.F.W. is a named inventor of intellectual property related to digeranyl bisphosphonate that is owned by the University of Iowa Research Foundation. He is a founder of Terpenoid Therapeutics, Inc., which has licensed this property.
Figures
References
-
- Ebetino F. H.; Hogan A. M. L.; Sun S.; Tsoumpra M. K.; Duan X.; Triffitt J. T.; Kwaasi A. A.; Dunford J. E.; Barnett B. L.; Oppermann U.; Lundy M. W.; Boyde A.; Kashemirov B. A.; McKenna C. E.; Russell R. G. G. The Relationship between the chemistry and biological activity of the bisphosphonates. Bone 2011, 49, 20–33. 10.1016/j.bone.2011.03.774. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

