Treatment of Surgical Site Infection in Posterior Lumbar Interbody Fusion
- PMID: 26713114
- PMCID: PMC4686387
- DOI: 10.4184/asj.2015.9.6.841
Treatment of Surgical Site Infection in Posterior Lumbar Interbody Fusion
Abstract
Study design: A retrospective observational and case control study.
Purpose: To identify appropriate treatment options according to the types of surgical site infections (SSI) in instrumented posterior lumbar interbody fusion (PLIF).
Overview of literature: There has been no agreement or consensus with regard to this matter.
Methods: Thirty-two consecutive SSIs were included and followed for more than one year. The elapsed time to diagnosis (ETD) according to the type of SSI was analyzed. The treatment options for each type and consequent clinical results were reviewed. The risk factors of removing the implants were analyzed.
Results: There were 6/32 (19%) superficial incisional, 6/32 (19%) deep incisional, and 20/32 (62%) organ/space infection cases (SII, DII, and O/SI, respectively) (p=0.002). ETD was 8.5±2.3 days in SII, 8.7±2.3 days in DII, and 164.5±131.1 days in O/SI (p=0.013). All cases of SII and DII retained implants and were treated by repeated irrigation and secondary closure. Among O/SIs, 10/20 were treated conservatively. Nine out of ten underwent posterior one stage simultaneous revision (POSSR) and in one case, the cage was removed anteriorly. Those who had ETDs longer than 3 months showed a significant risk of implant removal (p=0.008, odds ratio [OR]=40.3). The Oswestry disability index (ODI) improved from 47.3% to 33.8% in SII, from 55.0% to 32.3% in DII, and from 53.4% to 42.1% in O/SI (p=0.002). There was no difference among the three groups (p=0.106); however, there was a partial correlation between ETD and final ODI (r=0.382, p=0.034).
Conclusions: Latent O/SI was the most common type of SSI in PLIF. In cases of SII and DII, early aggressive wound management and secondary closure was effective and implant removal was not necessary. In some cases of O/SI, implant removal was unavoidable. However, implant removal could be averted by an earlier diagnosis. POSSR was feasible and safe. Functional outcomes were improved; however, disability increased as ETD increased.
Keywords: Implant removal; Posterior lumbar interbody fusion; Posterior one stage simultaneous revision; Surgical site infection; Treatment.
Conflict of interest statement
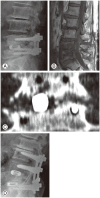
Figures

Similar articles
-
Particular Features of Surgical Site Infection in Posterior Lumbar Interbody Fusion.Clin Orthop Surg. 2015 Sep;7(3):337-43. doi: 10.4055/cios.2015.7.3.337. Epub 2015 Aug 13. Clin Orthop Surg. 2015. PMID: 26330956 Free PMC article.
-
Treatment strategy for surgical site infection post posterior lumbar interbody fusion: A retrospective study.J Orthop. 2022 Mar 18;31:40-44. doi: 10.1016/j.jor.2022.03.004. eCollection 2022 May-Jun. J Orthop. 2022. PMID: 35368734 Free PMC article.
-
Epidural abscess and discitis complicating instrumented posterior lumbar interbody fusion: a case report.Spine (Phila Pa 1976). 2004 Dec 1;29(23):E542-6. doi: 10.1097/01.brs.0000146802.38753.38. Spine (Phila Pa 1976). 2004. PMID: 15564903
-
Reduction in adjacent-segment degeneration after multilevel posterior lumbar interbody fusion with proximal DIAM implantation.J Neurosurg Spine. 2015 Aug;23(2):190-6. doi: 10.3171/2014.12.SPINE14666. Epub 2015 May 1. J Neurosurg Spine. 2015. PMID: 25932598
-
Risk factors for cage retropulsion after lumbar interbody fusion surgery: Series of cases and literature review.Int J Surg. 2016 Jun;30:56-62. doi: 10.1016/j.ijsu.2016.04.025. Epub 2016 Apr 21. Int J Surg. 2016. PMID: 27107661 Review.
Cited by
-
Adjacent Segment Infection after Lumbar Fusion: A Case Report and the Literature Review.Case Rep Orthop. 2020 Jan 20;2020:2163909. doi: 10.1155/2020/2163909. eCollection 2020. Case Rep Orthop. 2020. PMID: 32395359 Free PMC article.
-
Osteoconductivity and neurotoxicity of silver-containing hydroxyapatite coating cage for spinal interbody fusion in rats.JOR Spine. 2022 Nov 29;6(1):e1236. doi: 10.1002/jsp2.1236. eCollection 2023 Mar. JOR Spine. 2022. PMID: 36994462 Free PMC article.
-
Revision Surgery for Postoperative Spondylodiscitis at Cage Level after Posterior Instrumented Fusion in the Lumbar Spine-Anterior Approach Is Not Absolutely Indicated.J Clin Med. 2020 Nov 26;9(12):3833. doi: 10.3390/jcm9123833. J Clin Med. 2020. PMID: 33256126 Free PMC article.
-
Construction and validation of a nomogram predictive model for assessing the risk of surgical site infections following posterior lumbar fusion surgery.Sci Rep. 2025 Jan 6;15(1):1023. doi: 10.1038/s41598-024-84174-w. Sci Rep. 2025. PMID: 39762306 Free PMC article.
-
What Factors Predict Failure of Nonsurgical Management of a Lumbar Surgical Site Infection?Int J Spine Surg. 2019 Jun 30;13(3):239-244. doi: 10.14444/6032. eCollection 2019 Jun. Int J Spine Surg. 2019. PMID: 31328087 Free PMC article.
References
-
- Fritzell P, Hagg O, Wessberg P, Nordwall A. Chronic low back pain and fusion: a comparison of three surgical techniques: a prospective multicenter randomized study from the Swedish lumbar spine study group. Spine (Phila Pa 1976) 2002;27:1131–1141. - PubMed
-
- Christensen FB, Hansen ES, Eiskjaer SP, et al. Circumferential lumbar spinal fusion with Brantigan cage versus posterolateral fusion with titanium Cotrel-Dubousset instrumentation: a prospective, randomized clinical study of 146 patients. Spine (Phila Pa 1976) 2002;27:2674–2683. - PubMed
-
- Schofferman J, Slosar P, Reynolds J, Goldthwaite N, Koestler M. A prospective randomized comparison of 270 degrees fusions to 360 degrees fusions (circumferential fusions) Spine (Phila Pa 1976) 2001;26:E207–E212. - PubMed
-
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20:271–274. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

