Quantifying Gleason scores with photoacoustic spectral analysis: feasibility study with human tissues
- PMID: 26713193
- PMCID: PMC4679253
- DOI: 10.1364/BOE.6.004781
Quantifying Gleason scores with photoacoustic spectral analysis: feasibility study with human tissues
Abstract
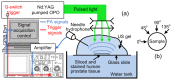
Gleason score is a highly prognostic factor for prostate cancer describing the microscopic architecture of the tumor tissue. The standard procedure for evaluating Gleason scores, namely biopsy, is to remove prostate tissue for observation under microscope. Currently, biopsies are guided by transrectal ultrasound (TRUS). Due to the low sensitivity of TRUS to prostate cancer (PCa), non-guided and saturated biopsies are frequently employed, unavoidably causing pain, damage to the normal prostate tissues and other complications. More importantly, due to the limited number of biopsy cores, current procedure could either miss early stage small tumors or undersample aggressive cancers. Photoacoustic (PA) measurement has the unique capability of evaluating tissue microscopic architecture information at ultrasonic resolution. By frequency domain analysis of the broadband PA signal, namely PA spectral analysis (PASA), the microscopic architecture within the assessed tissue can be quantified. This study investigates the feasibility of evaluating Gleason scores by PASA. Simulations with the classic Gleason patterns and experiment measurements from human PCa tissues have demonstrated strong correlation between the PASA parameters and the Gleason scores.
Keywords: (110.5125) Photoacoustics; (120.3890) Medical optics instrumentation; (170.6510) Spectroscopy, tissue diagnostics; (170.6935) Tissue characterization.
Figures




References
-
- Andriole G. L., Crawford E. D., Grubb R. L., 3rd, Buys S. S., Chia D., Church T. R., Fouad M. N., Isaacs C., Kvale P. A., Reding D. J., Weissfeld J. L., Yokochi L. A., O’Brien B., Ragard L. R., Clapp J. D., Rathmell J. M., Riley T. L., Hsing A. W., Izmirlian G., Pinsky P. F., Kramer B. S., Miller A. B., Gohagan J. K., Prorok P. C., “Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up,” J. Natl. Cancer Inst. 104(2), 125–132 (2012).10.1093/jnci/djr500 - DOI - PMC - PubMed
-
- Andriole G. L., Crawford E. D., Grubb R. L., 3rd, Buys S. S., Chia D., Church T. R., Fouad M. N., Gelmann E. P., Kvale P. A., Reding D. J., Weissfeld J. L., Yokochi L. A., O’Brien B., Clapp J. D., Rathmell J. M., Riley T. L., Hayes R. B., Kramer B. S., Izmirlian G., Miller A. B., Pinsky P. F., Prorok P. C., Gohagan J. K., Berg C. D., “Mortality results from a randomized prostate-cancer screening trial,” N. Engl. J. Med. 360(13), 1310–1319 (2009).10.1056/NEJMoa0810696 - DOI - PMC - PubMed
-
- Chou R., Croswell J. M., Dana T., Bougatsos C., Blazina I., Fu R., Gleitsmann K., Koenig H. C., Lam C., Maltz A., Rugge J. B., Lin K., “Screening for prostate cancer: a review of the evidence for the U.S. Preventive Services Task Force,” Ann. Intern. Med. 155(11), 762–771 (2011).10.7326/0003-4819-155-11-201112060-00375 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
