Aurora A phosphorylation of WD40-repeat protein 62 in mitotic spindle regulation
- PMID: 26713495
- PMCID: PMC4943692
- DOI: 10.1080/15384101.2015.1127472
Aurora A phosphorylation of WD40-repeat protein 62 in mitotic spindle regulation
Abstract
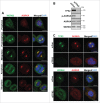
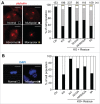
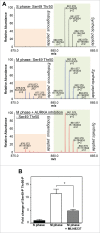
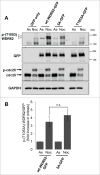
Mitotic spindle organization is regulated by centrosomal kinases that potentiate recruitment of spindle-associated proteins required for normal mitotic progress including the microcephaly protein WD40-repeat protein 62 (WDR62). WDR62 functions underlie normal brain development as autosomal recessive mutations and wdr62 loss cause microcephaly. Here we investigate the signaling interactions between WDR62 and the mitotic kinase Aurora A (AURKA) that has been recently shown to cooperate to control brain size in mice. The spindle recruitment of WDR62 is closely correlated with increased levels of AURKA following mitotic entry. We showed that depletion of TPX2 attenuated WDR62 localization at spindle poles indicating that TPX2 co-activation of AURKA is required to recruit WDR62 to the spindle. We demonstrated that AURKA activity contributed to the mitotic phosphorylation of WDR62 residues Ser49 and Thr50 and phosphorylation of WDR62 N-terminal residues was required for spindle organization and metaphase chromosome alignment. Our analysis of several MCPH-associated WDR62 mutants (V65M, R438H and V1314RfsX18) that are mislocalized in mitosis revealed that their interactions and phosphorylation by AURKA was substantially reduced consistent with the notion that AURKA is a key determinant of WDR62 spindle recruitment. Thus, our study highlights the role of AURKA signaling in the spatiotemporal control of WDR62 at spindle poles where it maintains spindle organization.
Keywords: aurora A; c-jun N-terminal kinase; mitosis; phosphorylation; primary microcephaly.
Figures


Comment in
-
Driving WDR62 to the pole.Cell Cycle. 2016 May 2;15(9):1180-1. doi: 10.1080/15384101.2016.1154374. Epub 2016 Apr 25. Cell Cycle. 2016. PMID: 27110649 Free PMC article. No abstract available.
References
-
- Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol 2004; 6:232-37; PMID:14767480; http://dx.doi.org/10.1038/ncb1102 - DOI - PubMed
-
- Compton DA. Spindle assembly in animal cells. Annu Rev Biochem 2000; 69:95-114; PMID:10966454; http://dx.doi.org/10.1146/annurev.biochem.69.1.95 - DOI - PubMed
-
- Zimmerman WC, Sillibourne J, Rosa J, Doxsey SJ. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol Biol Cell 2004; 15:3642-57; PMID:15146056; http://dx.doi.org/10.1091/mbc.E03-11-0796 - DOI - PMC - PubMed
-
- Oshimori N, Li X, Ohsugi M, Yamamoto T. Cep72 regulates the localization of key centrosomal proteins and proper bipolar spindle formation. EMBO J 2009; 28:2066-76; PMID:19536135; http://dx.doi.org/10.1038/emboj.2009.161 - DOI - PMC - PubMed
-
- Bornens M. The centrosome in cells and organisms. Science 2012; 335:422-6; PMID:22282802; http://dx.doi.org/10.1126/science.1209037 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
