Screening for Human Papillomavirus-Associated Cervical Disease in HIV-Infected Women
- PMID: 26713504
- PMCID: PMC6148917
Screening for Human Papillomavirus-Associated Cervical Disease in HIV-Infected Women
Abstract
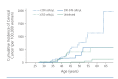
HIV-infected women have higher rates of persistence of human papillomavirus (HPV) infection, of abnormal cervical cytology results, and of cervical cancer than uninfected women. It is currently recommended that HIV-infected, sexually active women have a Papanicolaou (Pap) test performed at the time of initial diagnosis of HIV infection, followed by annual Pap testing if the previous test result is normal. Women whose test results show abnormalities greater than atypical squamous cells of undetermined significance (ASCUS) should be referred for colposcopy. Those with ASCUS should undergo immediate colposcopy or repeat cervical cytology in 6 months to 12 months, and those whose repeat cervical cytology results show ASCUS or greater abnormalities should undergo colposcopy. Recent findings indicate that screening intervals can be lengthened for HIV-infected women whose Pap test results are persistently normal and who are engaged in routine care, and that HPV DNA testing may have a role in screening. This article summarizes a presentation by Marla J. Keller, MD, at the IAS-USA continuing education program, Improving the Management of HIV Disease, held in Atlanta, Georgia, in March 2015.
Conflict of interest statement
Financial affiliations in the past 12 months: Dr. Keller has received provision of medicines, equipment, and administrative support from Gilead Sciences, Inc.
Figures
Similar articles
-
Effect of HIV infection on atypical squamous cells of undetermined significance.Clin Infect Dis. 2006 Mar 15;42(6):855-61. doi: 10.1086/500404. Epub 2006 Feb 10. Clin Infect Dis. 2006. PMID: 16477565
-
Randomized controlled trial of human papillomavirus testing versus Pap cytology in the primary screening for cervical cancer precursors: design, methods and preliminary accrual results of the Canadian cervical cancer screening trial (CCCaST).Int J Cancer. 2006 Aug 1;119(3):615-23. doi: 10.1002/ijc.21897. Int J Cancer. 2006. PMID: 16572425 Clinical Trial.
-
[The role of colposcopy in Population Program of Cervical Cancer Early Detection].Ginekol Pol. 2007 Sep;78(9):719-22. Ginekol Pol. 2007. PMID: 18159827 Polish.
-
Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries.Vaccine. 2008 Aug 19;26 Suppl 10:K29-41. doi: 10.1016/j.vaccine.2008.06.019. Vaccine. 2008. PMID: 18847555 Review.
-
Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer.Vaccine. 2012 Nov 20;30 Suppl 5:F88-99. doi: 10.1016/j.vaccine.2012.06.095. Vaccine. 2012. PMID: 23199969 Review.
Cited by
-
A review of screening strategies for cervical cancer in human immunodeficiency virus-positive women in sub-Saharan Africa.Int J Womens Health. 2017 Feb 2;9:69-79. doi: 10.2147/IJWH.S103868. eCollection 2017. Int J Womens Health. 2017. PMID: 28203108 Free PMC article. Review.
References
-
- Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC, Jr. Human papillomavirus infection in women infected with the human immunodeficiency virus. N Engl J Med. 1997;337(19):1343-1349. - PubMed
-
- Minkoff H, Feldman J, Dehovitz J, Landesman S, Burk R. A longitudinal study of human papillomavirus carriage in human immunodeficiency virus-infected and human immunodeficiency virus uninfected women. Am J Obstet Gynecol. 1998;178(5):982-986. - PubMed
-
- Massad LS, Seaberg EC, Wright RL, et al. Squamous cervical lesions in women with human immunodeficiency virus: long-term follow-up. Obstet Gynecol. 2008;111(6):1388-1393. - PubMed
-
- Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000; 92(18):1500-1510. - PubMed