Periplasmic Acid Stress Increases Cell Division Asymmetry (Polar Aging) of Escherichia coli
- PMID: 26713733
- PMCID: PMC4694651
- DOI: 10.1371/journal.pone.0144650
Periplasmic Acid Stress Increases Cell Division Asymmetry (Polar Aging) of Escherichia coli
Abstract
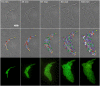
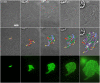
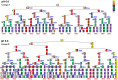
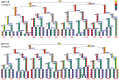
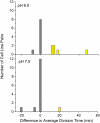
Under certain kinds of cytoplasmic stress, Escherichia coli selectively reproduce by distributing the newer cytoplasmic components to new-pole cells while sequestering older, damaged components in cells inheriting the old pole. This phenomenon is termed polar aging or cell division asymmetry. It is unknown whether cell division asymmetry can arise from a periplasmic stress, such as the stress of extracellular acid, which is mediated by the periplasm. We tested the effect of periplasmic acid stress on growth and division of adherent single cells. We tracked individual cell lineages over five or more generations, using fluorescence microscopy with ratiometric pHluorin to measure cytoplasmic pH. Adherent colonies were perfused continually with LBK medium buffered at pH 6.00 or at pH 7.50; the external pH determines periplasmic pH. In each experiment, cell lineages were mapped to correlate division time, pole age and cell generation number. In colonies perfused at pH 6.0, the cells inheriting the oldest pole divided significantly more slowly than the cells inheriting the newest pole. In colonies perfused at pH 7.50 (near or above cytoplasmic pH), no significant cell division asymmetry was observed. Under both conditions (periplasmic pH 6.0 or pH 7.5) the cells maintained cytoplasmic pH values at 7.2-7.3. No evidence of cytoplasmic protein aggregation was seen. Thus, periplasmic acid stress leads to cell division asymmetry with minimal cytoplasmic stress.
Conflict of interest statement
Figures
References
-
- Johnson LR, Mangel M. Life histories and the evolution of aging in bacteria and other single-celled organisms. Mech Ageing and Dev. 2006;127(10):786–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

