Evidence of β-Cell Dedifferentiation in Human Type 2 Diabetes
- PMID: 26713822
- PMCID: PMC4803182
- DOI: 10.1210/jc.2015-2860
Evidence of β-Cell Dedifferentiation in Human Type 2 Diabetes
Abstract
Context: Diabetes is associated with a deficit of insulin-producing β-cells. Animal studies show that β-cells become dedifferentiated in diabetes, reverting to a progenitor-like stage, and partly converting to other endocrine cell types.
Objective: To determine whether similar processes occur in human type 2 diabetes, we surveyed pancreatic islets from 15 diabetic and 15 nondiabetic organ donors.
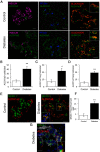
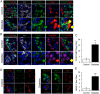
Design: We scored dedifferentiation using markers of endocrine lineage, β-cell-specific transcription factors, and a newly identified endocrine progenitor cell marker, aldehyde dehydrogenase 1A3.
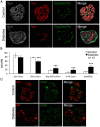
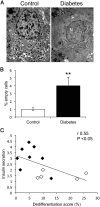
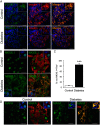
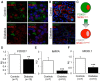
Results: By these criteria, dedifferentiated cells accounted for 31.9% of β-cells in type 2 diabetics vs 8.7% in controls, and for 16.8% vs 6.5% of all endocrine cells (P < .001). The number of aldehyde dehydrogenase 1A3-positive/hormone-negative cells was 3-fold higher in diabetics compared with controls. Moreover, β-cell-specific transcription factors were ectopically found in glucagon- and somatostatin-producing cells of diabetic subjects.
Conclusions: The data support the view that pancreatic β-cells become dedifferentiated and convert to α- and δ-"like" cells in human type 2 diabetes. The findings should prompt a reassessment of goals in the prevention and treatment of β-cell dysfunction.
Figures
References
-
- Ferrannini E. The stunned β cell: a brief history. Cell Metab. 2010;11:349–352. - PubMed
-
- Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. β-Cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med. 1998;15:290–296. - PubMed
-
- Savage PJ, Bennion LJ, Flock EV, et al. Diet-induced improvement of abnormalities in insulin and glucagon secretion and in insulin receptor binding in diabetes mellitus. J Clin Endocrinol Metab. 1979;48:999–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

