Roles of Asp179 and Glu270 in ADP-Ribosylation of Actin by Clostridium perfringens Iota Toxin
- PMID: 26713879
- PMCID: PMC4699905
- DOI: 10.1371/journal.pone.0145708
Roles of Asp179 and Glu270 in ADP-Ribosylation of Actin by Clostridium perfringens Iota Toxin
Abstract
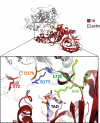
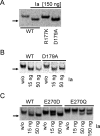
Clostridium perfringens iota toxin is a binary toxin composed of the enzymatically active component Ia and receptor binding component Ib. Ia is an ADP-ribosyltransferase, which modifies Arg177 of actin. The previously determined crystal structure of the actin-Ia complex suggested involvement of Asp179 of actin in the ADP-ribosylation reaction. To gain more insights into the structural requirements of actin to serve as a substrate for toxin-catalyzed ADP-ribosylation, we engineered Saccharomyces cerevisiae strains, in which wild type actin was replaced by actin variants with substitutions in residues located on the Ia-actin interface. Expression of the actin mutant Arg177Lys resulted in complete resistance towards Ia. Actin mutation of Asp179 did not change Ia-induced ADP-ribosylation and growth inhibition of S. cerevisiae. By contrast, substitution of Glu270 of actin inhibited the toxic action of Ia and the ADP-ribosylation of actin. In vitro transcribed/translated human β-actin confirmed the crucial role of Glu270 in ADP-ribosylation of actin by Ia.
Conflict of interest statement
Figures



Similar articles
-
ADP-ribosylation of skeletal muscle and non-muscle actin by Clostridium perfringens iota toxin.Eur J Biochem. 1988 Jan 15;171(1-2):225-9. doi: 10.1111/j.1432-1033.1988.tb13780.x. Eur J Biochem. 1988. PMID: 2892681
-
ADP-ribosylation of Drosophila indirect-flight-muscle actin and arthrin by Clostridium botulinum C2 toxin and Clostridium perfringens iota toxin.Biochem J. 1993 Apr 15;291 ( Pt 2)(Pt 2):409-12. doi: 10.1042/bj2910409. Biochem J. 1993. PMID: 8484722 Free PMC article.
-
De-ADP-ribosylation actin by Clostridium perfringens iota-toxin and Clostridium botulinum C2 toxin.Eur J Biochem. 1990 Sep 24;192(3):723-7. doi: 10.1111/j.1432-1033.1990.tb19282.x. Eur J Biochem. 1990. PMID: 2145159
-
Clostridial ADP-ribosylating toxins: effects on ATP and GTP-binding proteins.Mol Cell Biochem. 1994 Sep;138(1-2):167-76. doi: 10.1007/BF00928459. Mol Cell Biochem. 1994. PMID: 7898461 Review.
-
Receptor-Binding and Uptake of Binary Actin-ADP-Ribosylating Toxins.Curr Top Microbiol Immunol. 2017;406:119-133. doi: 10.1007/82_2016_46. Curr Top Microbiol Immunol. 2017. PMID: 27817176 Review.
Cited by
-
Crystal structure and structure-based mutagenesis of actin-specific ADP-ribosylating toxin CPILE-a as novel enterotoxin.PLoS One. 2017 Feb 15;12(2):e0171278. doi: 10.1371/journal.pone.0171278. eCollection 2017. PLoS One. 2017. PMID: 28199340 Free PMC article.
-
Individual and group format adjunct therapy on social emotional skills for adolescent inpatients with severe and complex eating disorders (CREST-A).Neuropsychiatr. 2021 Dec;35(4):163-176. doi: 10.1007/s40211-020-00375-5. Epub 2020 Nov 30. Neuropsychiatr. 2021. PMID: 33252714 English.
-
Actin activates Pseudomonas aeruginosa ExoY nucleotidyl cyclase toxin and ExoY-like effector domains from MARTX toxins.Nat Commun. 2016 Dec 5;7:13582. doi: 10.1038/ncomms13582. Nat Commun. 2016. PMID: 27917880 Free PMC article.
-
The Actin-Binding Prolyl-Isomerase Par17 Sustains Its Substrate Selectivity by Interdomain Allostery.Proteins. 2025 Sep;93(9):1481-1497. doi: 10.1002/prot.26807. Epub 2025 Mar 12. Proteins. 2025. PMID: 40071814 Free PMC article.
-
Structure and activation mechanism of the Makes caterpillars floppy 1 toxin.Nat Commun. 2023 Dec 12;14(1):8226. doi: 10.1038/s41467-023-44069-2. Nat Commun. 2023. PMID: 38086871 Free PMC article.
References
-
- Marvaud JC, Stiles BG, Chenal A, Gillet D, Gibert M, Smith LA, et al. Clostridium perfringens iota toxin. Mapping of the Ia domain involved in docking with Ib and cellular internalization. J Biol Chem. 2002;277:43659–43666. - PubMed
-
- Barth H, Blöcker D, Behlke J, Bergsma-Schutter W, Brisson A, Benz R, et al. Cellular uptake of Clostridium botulinum C2 toxin requires oligomerization and acidification. J Biol Chem. 2000;275:18704–18711. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

