Durability and Effectiveness of Maraviroc-Containing Regimens in HIV-1-Infected Individuals with Virological Failure in Routine Clinical Practice
- PMID: 26714012
- PMCID: PMC4695083
- DOI: 10.1371/journal.pone.0144746
Durability and Effectiveness of Maraviroc-Containing Regimens in HIV-1-Infected Individuals with Virological Failure in Routine Clinical Practice
Abstract
Introduction: Limited data are available on the durability and effectiveness of maraviroc in routine clinical practice. We assessed the durability of maraviroc-containing regimens during a 30-month period, as well as their immunovirological and clinical efficacy, according to viral tropism in treatment-experienced individuals with viral load (VL) >50 copies/ml in the French Hospital Database on HIV.
Methods: Virological success was defined as VL<50 copies/ml, immunological success as a confirmed increase of at least 100 CD4 cells/mm3 measured twice at least one month apart, and clinical failure as hospitalization for a non-AIDS event, an AIDS event, or death. Multivariable Cox regression models adjusted for potential confounders were used to assess the influence of viral tropism on durability, the immunovirological responses, and clinical outcome.
Results: 356 individuals started maraviroc with VL>50 copies/ml of whom 223 harbored R5 viruses, 44 non-R5 viruses and 89 viruses of unknown tropism. Individuals with non-R5 viruses were more likely than individuals with R5 viruses to discontinue maraviroc (75% vs 34%, p<0.0001). At 30 months, the estimated rates of virological and immunological success were respectively 89% and 51% in individuals with R5 viruses and 48% and 23% in individuals with non-R5 viruses. In multivariable analysis, non-R5 viruses were associated with a lower likelihood of both virological success (hazard ratio (HR): 0.42; 95% confidence interval (CI), 0.25-0.70) and immunological success (HR: 0.37; 95% CI, 0.18-0.77). No difference in clinical outcome was found between individuals with R5 and non-R5 viruses. The effectiveness of maraviroc-containing regimens in individuals with unknown viral tropism was not significantly different from that in individuals with R5 viruses. A limitation of the study is the absence of genotypic susceptibility score.
Conclusion: In this observational study, maraviroc-containing regimens yielded high rates of viral suppression and immunological responses in individuals with R5 viruses in whom prior regimens had failed.
Conflict of interest statement
Figures


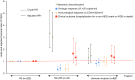
References
-
- Phillips AN, Dunn D, Sabin C, Pozniak A, Matthias R, Geretti AM, et al. Long term probability of detection of HIV-1 drug resistance after starting antiretroviral therapy in routine clinical practice. AIDS 2005;19:487–94. - PubMed
-
- Vray M, Meynard JL, Dalban C, Morand-Joubert L, Clavel F, Brun-Vézinet F, et al. Predictors of the virological response to a change in the antiretroviral treatment regimen in HIV-1-infected individuals enrolled in a randomized trial comparing genotyping, phenotyping and standard of care (Narval trial, ANRS088). Antiviral Therapy 2003; 8:427–434. - PubMed
-
- Panel on Clinical pratices for treatment of HIV infection. Guidelines for the use of antiretroviral agents in HIV-1 Infected Adults and Adolescents: Aidsinfo, Feb 12, 2013. Available: http://www.aidsinfo.nih.gov/guidelines/archive/adult-and-adolescent-guid...
-
- Fätkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AIM, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in individuals infected with HIV-1. Nature Medicine 2005;11:1170–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

