Discovery of the gray phenotype and white-gray-opaque tristable phenotypic transitions in Candida dubliniensis
- PMID: 26714067
- PMCID: PMC4871672
- DOI: 10.1080/21505594.2015.1135287
Discovery of the gray phenotype and white-gray-opaque tristable phenotypic transitions in Candida dubliniensis
Abstract
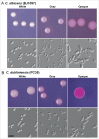
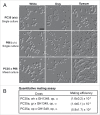
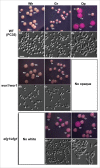
Candida dubliniensis is closely related to Candida albicans, a major causative agent of candidiasis, and is primarily associated with oral colonization and infection in human immunodeficiency virus (HIV)-positive patients. Despite the high similarity of genomic and phenotypic features between the 2 species, C. dubliniensis is much less virulent and less prevalent than C. albicans. The ability to change morphological phenotypes is a striking feature of Candida species and is linked to virulence. In this study, we report a novel phenotype, the gray phenotype, in C. dubliniensis. Together with the previously reported white and opaque cell types, the gray phenotype forms a tristable phenotypic switching system in C. dubliniensis that is similar to the white-gray-opaque tristable switching system in C. albicans. Gray cells of C. dubliniensis are similar to their counterparts in C. albicans in terms of several biological aspects including cellular morphology, mating competence, and genetic regulatory mechanisms. However, the gray phenotypes of the 2 species have some distinguishing features. For example, the secreted aspartyl protease (Sap) activity is induced by bovine serum albumin (BSA) in gray cells of C. albicans, but not in gray cells of C. dubliniensis. Taken together, our results demonstrate that the biological features and regulatory mechanisms of white-gray-opaque tristable transitions are largely conserved in the 2 pathogenic Candida species.
Keywords: Candida dubliniensis; Efg1; Wor1; pathogenesis; phenotypic switching.
Figures



Comment in
-
Gray phenotype: Enhanced fitness strategy for Candida dubliniensis?Virulence. 2016 Apr 2;7(3):211-3. doi: 10.1080/21505594.2016.1142641. Epub 2016 Jan 19. Virulence. 2016. PMID: 26786843 Free PMC article. No abstract available.
Similar articles
-
Discovery of a "white-gray-opaque" tristable phenotypic switching system in candida albicans: roles of non-genetic diversity in host adaptation.PLoS Biol. 2014 Apr 1;12(4):e1001830. doi: 10.1371/journal.pbio.1001830. eCollection 2014 Apr. PLoS Biol. 2014. PMID: 24691005 Free PMC article.
-
The gray phenotype and tristable phenotypic transitions in the human fungal pathogen Candida tropicalis.Fungal Genet Biol. 2016 Aug;93:10-6. doi: 10.1016/j.fgb.2016.05.006. Epub 2016 May 28. Fungal Genet Biol. 2016. PMID: 27246518
-
Role of the N-acetylglucosamine kinase (Hxk1) in the regulation of white-gray-opaque tristable phenotypic transitions in C. albicans.Fungal Genet Biol. 2016 Jul;92:26-32. doi: 10.1016/j.fgb.2016.05.001. Epub 2016 May 3. Fungal Genet Biol. 2016. PMID: 27153757
-
Candida dubliniensis: ten years on.FEMS Microbiol Lett. 2005 Dec 1;253(1):9-17. doi: 10.1016/j.femsle.2005.09.015. Epub 2005 Sep 26. FEMS Microbiol Lett. 2005. PMID: 16213674 Review.
-
Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans.FEMS Yeast Res. 2004 Jan;4(4-5):369-76. doi: 10.1016/S1567-1356(03)00240-X. FEMS Yeast Res. 2004. PMID: 14734017 Review.
Cited by
-
The changes of antifungal susceptibilities caused by the phenotypic switching of Candida species in 229 patients with vulvovaginal candidiasis.J Clin Lab Anal. 2019 Jan;33(1):e22644. doi: 10.1002/jcla.22644. Epub 2018 Aug 12. J Clin Lab Anal. 2019. PMID: 30101450 Free PMC article.
-
Phenotypes characterization and ABC genotypes distribution of clinical Candida albicans isolates.J Clin Lab Anal. 2023 Apr;37(7):e24888. doi: 10.1002/jcla.24888. Epub 2023 Apr 25. J Clin Lab Anal. 2023. PMID: 37096939 Free PMC article.
-
Parasexuality of Candida Species.Front Cell Infect Microbiol. 2021 Dec 13;11:796929. doi: 10.3389/fcimb.2021.796929. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34966696 Free PMC article. Review.
-
Phenotypic switching in Candida tropicalis alters host-pathogen interactions in a Galleria mellonella infection model.Sci Rep. 2019 Aug 29;9(1):12555. doi: 10.1038/s41598-019-49080-6. Sci Rep. 2019. PMID: 31467372 Free PMC article.
-
Gray phenotype: Enhanced fitness strategy for Candida dubliniensis?Virulence. 2016 Apr 2;7(3):211-3. doi: 10.1080/21505594.2016.1142641. Epub 2016 Jan 19. Virulence. 2016. PMID: 26786843 Free PMC article. No abstract available.
References
-
- Pfaller MA. Epidemiology of candidiasis. J Hosp Infect 1995; 30 Suppl:329-38; PMID:7560969; http://dx.doi.org/10.1016/0195-6701(95)90036-5 - DOI - PubMed
-
- Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev 2007; 71:348-76; PMID:17554048; http://dx.doi.org/10.1128/MMBR.00009-06 - DOI - PMC - PubMed
-
- Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 2007; 20:133-63; PMID:17223626; http://dx.doi.org/10.1128/CMR.00029-06 - DOI - PMC - PubMed
-
- Whiteway M, Bachewich C. Morphogenesis in Candida albicans. Annu Rev Microbiol 2007; 61:529-53; PMID:17506678; http://dx.doi.org/10.1146/annurev.micro.61.080706.093341 - DOI - PMC - PubMed
-
- Huang G. Regulation of phenotypic transitions in the fungal pathogen Candida albicans. Virulence 2012; 3; 251-61; PMID:22546903; http://dx.doi.org/10.4161/viru.20010 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
