Mutated Leguminous Lectin Containing a Heparin-Binding like Motif in a Carbohydrate-Binding Loop Specifically Binds to Heparin
- PMID: 26714191
- PMCID: PMC4701002
- DOI: 10.1371/journal.pone.0145834
Mutated Leguminous Lectin Containing a Heparin-Binding like Motif in a Carbohydrate-Binding Loop Specifically Binds to Heparin
Abstract
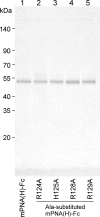
We previously introduced random mutations in the sugar-binding loops of a leguminous lectin and screened the resulting mutated lectins for novel specificities using cell surface display. Screening of a mutated peanut agglutinin (PNA), revealed a mutated PNA with a distinct preference for heparin. Glycan microarray analyses using the mutated lectin fused to the Fc region of human immunoglobulin, revealed that a particular sulfated glycosaminoglycan (GAG), heparin, had the highest binding affinity for mutated PNA among 97 glycans tested, although wild-type PNA showed affinity towards Galβ1-3GalNAc and similar galactosylated glycans. Further analyses of binding specificity using an enzyme-linked immunoadsorbent assay demonstrated that the mutated PNA specifically binds to heparin, and weakly to de-2-O-sulfated heparin, but not to other GAG chains including de-6-O-sulfated and de-N-sulfated heparins. The mutated PNA had six amino acid substitutions within the eight amino acid-long sugar-binding loop. In this loop, the heparin-binding like motif comprised three arginine residues at positions 124, 128, and 129, and a histidine at position 125 was present. Substitution of each arginine or histidine residue to alanine reduced heparin-binding ability, indicating that all of these basic amino acid residues contributed to heparin binding. Inhibition assay demonstrated that heparin and dextran sulfate strongly inhibited mutated PNA binding to heparin in dose-dependent manner. The mutated PNA could distinguish between CHO cells and proteoglycan-deficient mutant cells. This is the first report establishing a novel leguminous lectin that preferentially binds to highly sulfated heparin and may provide novel GAG-binding probes to distinguish between heterogeneous GAG repeating units.
Conflict of interest statement
Figures


Similar articles
-
Mammalian Cell Surface Display as a Novel Method for Developing Engineered Lectins with Novel Characteristics.Biomolecules. 2015 Jul 20;5(3):1540-62. doi: 10.3390/biom5031540. Biomolecules. 2015. PMID: 26287256 Free PMC article.
-
Molecular basis of recognition by Gal/GalNAc specific legume lectins: influence of Glu 129 on the specificity of peanut agglutinin (PNA) towards C2-substituents of galactose.Glycobiology. 1998 Oct;8(10):1007-12. doi: 10.1093/glycob/8.10.1007. Glycobiology. 1998. PMID: 9719681
-
Architecture of the sugar binding sites in carbohydrate binding proteins--a computer modeling study.Int J Biol Macromol. 1998 Nov;23(4):295-307. doi: 10.1016/s0141-8130(98)00056-7. Int J Biol Macromol. 1998. PMID: 9849627
-
Differential contributions of recognition factors of two plant lectins -Amaranthus caudatus lectin and Arachis hypogea agglutinin, reacting with Thomsen-Friedenreich disaccharide (Galbeta1-3GalNAcalpha1-Ser/Thr).Biochimie. 2008 Nov-Dec;90(11-12):1769-80. doi: 10.1016/j.biochi.2008.08.001. Epub 2008 Sep 3. Biochimie. 2008. PMID: 18809460
-
Lectin-immobilized fluorescent nanospheres for targeting to colorectal cancer from a physicochemical perspective.Curr Drug Discov Technol. 2011 Dec 1;8(4):367-78. doi: 10.2174/157016311798109407. Curr Drug Discov Technol. 2011. PMID: 21644921 Review.
Cited by
-
Glycosaminoglycan microarrays for studying glycosaminoglycan-protein systems.Carbohydr Polym. 2024 Jul 1;335:122106. doi: 10.1016/j.carbpol.2024.122106. Epub 2024 Mar 29. Carbohydr Polym. 2024. PMID: 38616080 Free PMC article. Review.
-
Origins of glycan selectivity in streptococcal Siglec-like adhesins suggest mechanisms of receptor adaptation.Nat Commun. 2022 May 18;13(1):2753. doi: 10.1038/s41467-022-30509-y. Nat Commun. 2022. PMID: 35585145 Free PMC article.
-
Lectin engineering: the possible and the actual.Interface Focus. 2019 Apr 6;9(2):20180068. doi: 10.1098/rsfs.2018.0068. Epub 2019 Feb 15. Interface Focus. 2019. PMID: 30842871 Free PMC article. Review.
-
Identification of mammalian glycoproteins with type-I LacdiNAc structures synthesized by the glycosyltransferase B3GALNT2.J Biol Chem. 2019 May 3;294(18):7433-7444. doi: 10.1074/jbc.RA118.006892. Epub 2019 Mar 21. J Biol Chem. 2019. PMID: 30898876 Free PMC article.
-
Angiotensin II receptor blockers and bone fracture in chronic kidney disease patients: the Fukuoka kidney disease Registry Study.Clin Exp Nephrol. 2023 Nov;27(11):919-927. doi: 10.1007/s10157-023-02385-3. Epub 2023 Jul 27. Clin Exp Nephrol. 2023. PMID: 37498346
References
-
- Sharon N, Lis H. Plant lecttins In: Sharon N, Lis H, editors. Lectins. 2nd ed. Dordrecht: Kluwer; 1993. pp. 179–198.
-
- Sharma V, Surolia A. Analyses of carbohydrate recognition by legume lectins: size of the combining site loops and their primary specificity. J Mol Biol. 1997;267: 433–445. - PubMed
-
- Liener IE, Sharon N, Goldstein IJ. Carbohydrate-binding specificity of lectins In: Liener IE, Sharon N, Goldstein IJ, editors. The lectins, properties, functions, and applications in biology and medicine. London: Academic press, 1986. pp. 43–49.
-
- Konami Y, Ishida C, Yamamoto K, Osawa T, Irimura T. A unique amino acid sequence involved in the putative carbohydrate-binding domain of a legume lectin specific for sialylated carbohydrate chains: primary sequence determination of Maackia amurensis hemagglutinin (MAH). J Biochem. 1994;115: 767–777. - PubMed
-
- Yamamoto K, Konami Y, Irimura T. Sialic acid-binding motif of Maackia amurensis lectins. J Biochem. 1997;121: 756–761. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

