Hypoxia Increases IGFBP-1 Phosphorylation Mediated by mTOR Inhibition
- PMID: 26714229
- PMCID: PMC4792228
- DOI: 10.1210/me.2015-1194
Hypoxia Increases IGFBP-1 Phosphorylation Mediated by mTOR Inhibition
Abstract
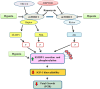
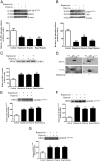
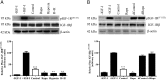
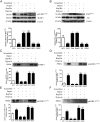
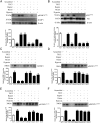
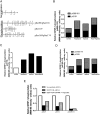
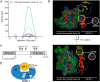
In fetal growth restriction (FGR), fetal growth is limited by reduced nutrient and oxygen supply. Insulin-like growth factor I (IGF-I) is a key regulator of fetal growth and IGF binding protein -1(IGFBP-1) is the principal regulator of fetal IGF-I bioavailability. Phosphorylation enhances IGFBP-1's affinity for IGF-I. Hypoxia induces IGFBP-1 hyperphosphorylation, markedly decreasing IGF-I bioavailability. We recently reported that fetal liver IGFBP-1 hyperphosphorylation is associated with inhibition of the mechanistic target of rapamycin (mTOR) in a nonhuman primate model of FGR. Here, we test the hypothesis that IGFBP-1 hyperphosphorylation in response to hypoxia is mediated by mTOR inhibition. We inhibited mTOR either by rapamycin or small interfering RNA (siRNA) targeting raptor (mTOR complex [mTORC]1) and/or rictor (mTORC2) in HepG2 cells cultured under hypoxia (1% O2) or basal (20% O2) conditions. Conversely, we activated mTORC1 or mTORC1+mTORC2 by silencing endogenous mTOR inhibitors (tuberous sclerosis complex 2/DEP-domain-containing and mTOR-interacting protein). Immunoblot analysis demonstrated that both hypoxia and inhibition of mTORC1 and/or mTORC2 induced similar degrees of IGFBP-1 phosphorylation at Ser101/119/169 and reduced IGF-I receptor autophosphorylation. Activation of mTORC1+mTORC2 or mTORC1 alone prevented IGFBP-1 hyperphosphorylation in response to hypoxia. Multiple reaction monitoring-mass spectrometry showed that rapamycin and/or hypoxia increased phosphorylation also at Ser98 and at a novel site Ser174. In silico structural analysis indicated that Ser174 was in close proximity to the IGF-binding site. Together, we demonstrate that signaling through the mTORC1 or mTORC2 pathway is sufficient to induce IGFBP-1 hyperphosphorylation in response to hypoxia. This study provides novel understanding of the cellular mechanism that controls fetal IGFBP-1 phosphorylation in hypoxia, and we propose that mTOR inhibition constitutes a mechanistic link between hypoxia, reduced IGF-I bioavailability and FGR.
Figures
References
-
- Eleftheriades M, Creatsas G, Nicolaides K. Fetal growth restriction and postnatal development. Ann NY Acad Sci. 2006;1092:319–330. - PubMed
-
- Kinzler WL, Vintzileos AM. Fetal growth restriction: a modern approach. Curr Opin Obstet Gynecol. 2008;20:125–131. - PubMed
-
- Ruijtenbeek K, Kessels LC, De Mey JG, Blanco CE. Chronic moderate hypoxia and protein malnutrition both induce growth retardation, but have distinct effects on arterial endothelium-dependent reactivity in the chicken embryo. Pediatr Res. 2003;53:573–579. - PubMed
-
- Randhawa R, Cohen P. The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab. 2005;86:84–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

