How to Capitalize on the Retest Effect in Future Trials on Huntington's Disease
- PMID: 26714284
- PMCID: PMC4703129
- DOI: 10.1371/journal.pone.0145842
How to Capitalize on the Retest Effect in Future Trials on Huntington's Disease
Abstract
The retest effect-improvement of performance on second exposure to a task-may impede the detection of cognitive decline in clinical trials for neurodegenerative diseases. We assessed the impact of the retest effect in Huntington's disease trials, and investigated its possible neutralization. We enrolled 54 patients in the Multicentric Intracerebral Grafting in Huntington's Disease (MIG-HD) trial and 39 in the placebo arm of the Riluzole trial in Huntington's Disease (RIL-HD). All were assessed with the Unified Huntington's Disease Rating Scale (UHDRS) plus additional cognitive tasks at baseline (A1), shortly after baseline (A2) and one year later (A3). We used paired t-tests to analyze the retest effect between A1 and A2. For each task of the MIG-HD study, we used a stepwise algorithm to design models predictive of patient performance at A3, which we applied to the RIL-HD trial for external validation. We observed a retest effect in most cognitive tasks. A decline in performance at one year was detected in 3 of the 15 cognitive tasks with A1 as the baseline, and 9 of the 15 cognitive tasks with A2 as the baseline. We also included the retest effect in performance modeling and showed that it facilitated performance prediction one year later for 14 of the 15 cognitive tasks. The retest effect may mask cognitive decline in patients with neurodegenerative diseases. The dual baseline can improve clinical trial design, and better prediction should homogenize patient groups, resulting in smaller numbers of participants being required.
Trial registration: ClinicalTrials.gov NCT00190450 NCT00277602.
Conflict of interest statement
Figures
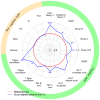
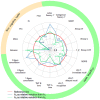
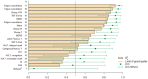
References
-
- Bates G, Tabrizi S, Jones L. Huntington’s Disease. 3rd ed Oxford: Oxford University Press; 2014.
-
- Bachoud-Lévi A-C, Maison P, Bartolomeo P, Boissé M-F, Dalla-Barba G, Ergis A-M, et al. Retest effects and cognitive decline in longitudinal follow-up of patients with early HD. Neurology. 2001;56(8):1052–8. - PubMed
-
- Salthouse TA, Schroeder DH, Ferrer E. Estimating retest effects in longitudinal assessments of cognitive functioning in adults between 18 and 60 years of age. Dev Psychol. 2004;40(5):813–22. - PubMed
-
- Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J Int Neuropsychol Soc. 2003;9(3):419–28. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

