Titanium Dioxide Nanoparticles Increase Superoxide Anion Production by Acting on NADPH Oxidase
- PMID: 26714308
- PMCID: PMC4699827
- DOI: 10.1371/journal.pone.0144829
Titanium Dioxide Nanoparticles Increase Superoxide Anion Production by Acting on NADPH Oxidase
Abstract
Titanium dioxide (TiO2) anatase nanoparticles (NPs) are metal oxide NPs commercialized for several uses of everyday life. However their toxicity has been poorly investigated. Cellular internalization of NPs has been shown to activate macrophages and neutrophils that contribute to superoxide anion production by the NADPH oxidase complex. Transmission electron micrososcopy images showed that the membrane fractions were close to the NPs while fluorescence indicated an interaction between NPs and cytosolic proteins. Using a cell-free system, we have investigated the influence of TiO2 NPs on the behavior of the NADPH oxidase. In the absence of the classical activator molecules of the enzyme (arachidonic acid) but in the presence of TiO2 NPs, no production of superoxide ions could be detected indicating that TiO2 NPs were unable to activate by themselves the complex. However once the NADPH oxidase was activated (i.e., by arachidonic acid), the rate of superoxide anion production went up to 140% of its value without NPs, this effect being dependent on their concentration. In the presence of TiO2 nanoparticles, the NADPH oxidase produces more superoxide ions, hence induces higher oxidative stress. This hyper-activation and the subsequent increase in ROS production by TiO2 NPs could participate to the oxidative stress development.
Conflict of interest statement
Figures


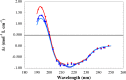
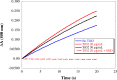


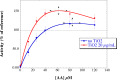

Similar articles
-
Band Alignment-Driven Oxidative Injury to the Skin by Anatase/Rutile Mixed-Phase Titanium Dioxide Nanoparticles Under Sunlight Exposure.Toxicol Sci. 2018 Jul 1;164(1):300-312. doi: 10.1093/toxsci/kfy088. Toxicol Sci. 2018. PMID: 29669021
-
Coexistence of silver and titanium dioxide nanoparticles: enhancing or reducing environmental risks?Aquat Toxicol. 2014 Sep;154:168-75. doi: 10.1016/j.aquatox.2014.05.020. Epub 2014 May 27. Aquat Toxicol. 2014. PMID: 24907921
-
Microglial cells (BV-2) internalize titanium dioxide (TiO2) nanoparticles: toxicity and cellular responses.Environ Sci Pollut Res Int. 2016 May;23(10):9690-9. doi: 10.1007/s11356-016-6190-7. Epub 2016 Feb 5. Environ Sci Pollut Res Int. 2016. PMID: 26846246
-
The inflammatory response to silver and titanium dioxide nanoparticles in the central nervous system.Nanomedicine (Lond). 2018 Jan;13(2):233-249. doi: 10.2217/nnm-2017-0270. Epub 2017 Dec 4. Nanomedicine (Lond). 2018. PMID: 29199887 Review.
-
Titanium Dioxide Nanoparticles: a Risk for Human Health?Mini Rev Med Chem. 2016;16(9):762-9. doi: 10.2174/1389557516666160321114341. Mini Rev Med Chem. 2016. PMID: 26996620 Review.
Cited by
-
Kinetic Effects of Transferrin-Conjugated Gold Nanoparticles on the Antioxidant Glutathione-Thioredoxin Pathway.Antioxidants (Basel). 2023 Aug 15;12(8):1617. doi: 10.3390/antiox12081617. Antioxidants (Basel). 2023. PMID: 37627612 Free PMC article.
-
Integration of metabolomics and transcriptomics in nanotoxicity studies.BMB Rep. 2018 Jan;51(1):14-20. doi: 10.5483/bmbrep.2018.51.1.237. BMB Rep. 2018. PMID: 29301609 Free PMC article.
-
Evaluation of specific and non-specific immune response of four vaccines for caseous lymphadenitis in sheep challenged.Vet World. 2018 Sep;11(9):1272-1276. doi: 10.14202/vetworld.2018.1272-1276. Epub 2018 Sep 17. Vet World. 2018. PMID: 30410233 Free PMC article.
-
Structural profiles of the full phagocyte NADPH oxidase unveiled by combining computational biology and experimental knowledge.J Biol Chem. 2024 Dec;300(12):107943. doi: 10.1016/j.jbc.2024.107943. Epub 2024 Oct 29. J Biol Chem. 2024. PMID: 39481598 Free PMC article.
-
Detection of Oxidative Stress Induced by Nanomaterials in Cells-The Roles of Reactive Oxygen Species and Glutathione.Molecules. 2021 Aug 4;26(16):4710. doi: 10.3390/molecules26164710. Molecules. 2021. PMID: 34443297 Free PMC article. Review.
References
-
- Niederberger M, Pinna N (2009) Metal oxide nanoparticles in organic solvents: synthesis, formation, assembly and application: Springer.
-
- Yuan Y, Ding J, Xu J, Deng J, Guo J (2010) TiO2 nanoparticles co-doped with silver and nitrogen for antibacterial application. J Nanosci Nanotechnol 10: 4868–4874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

